Hydrogen inhibits Lung Carcer progressionScientific Research
Hydrogen therapy inhibits lung cancer growth
Hydrogen therapy inhibits the growth and spread of lung cancer cells. Researchers at Hebei Medical University in China have focused on hydrogen as a potential therapeutic agent known for its safety profile and non-toxic characteristics. Their study reveals that hydrogen may play a key role in halting the progression of lung cancer.
The methodology of this study includes both in vitro and in vivo approaches. One works at the cellular level and the other at the whole organism level. The aim is to cover the full spectrum of effects of hydrogen. Chinese scientists have conducted experiments with lung cancer cell lines such as a five four nine and hash one nine seven five, exposing them to different concentrations of hydrogen. These tests aim to assess the effects of hydrogen on cell behavior, such as growth rate, invasive potential and survival mechanisms.
Hydrogen therapy demonstrates a powerful anti-cancer effect.
In addition, tissue from mice has been analyzed to assess the therapeutic efficacy of hydrogen inhalation on tumor growth and immune system response, ensuring that the findings are relevant and could potentially be incorporated into cancer treatment protocols.
Here are specific results from the study. Hydrogen treatment limited the rate at which both the number and invasiveness of lung cancer cells increased, suggesting a potent anti-cancer effect. In animal models, hydrogen inhalation resulted in a marked reduction in tumor size. It also apparently increased the capacity of the immune system to engage and destroy cancer cells. The results suggest that hydrogen not only acts directly against tumors, but may also facilitate an enhanced immune response, creating a two-pronged approach to fighting lung cancer.
Ultimately, what is the potential of hydrogen therapy? It demonstrates a dual action – on the one hand it directly suppresses tumor cells, and on the other it stimulates the immune system. Research conducted at Hebei Medical University offers evidence to support the integration of hydrogen therapy into existing cancer treatment protocols, positioning it as a safe and effective option that can significantly improve clinical outcomes for lung cancer patients. Currently, hydrogen research is in its infancy, but results show strong potential.
The Original Article:
original title: Hydrogen gas represses the progression of lung cancer via down-regulating CD47
DOI: 10.1042/BSR20192761Abstract
Hydrogen (H2) has been found to exert antitumor effects in several types of cancer, but its molecular mechanisms remain largely unknown. In our previous study, our project group found that H2 may reduce the expression of CD47 in lung cancer A549 cells through next-generation sequencing, suggesting that CD47 may be involved in H2-mediated lung cancer suppression. Therefore, this study aimed to investigate the effect of CD47 on H2-induced lung cancer suppression. Western blotting and real-time PCR (RT-PCR) assays were used to detect protein and mRNA levels, respectively. Cell proliferation, invasion, migration and apoptosis were detected using Cell Counting Kit-8 (CCK-8), Transwell chamber, wound healing and flow cytometry, respectively. The results showed that H2 treatment resulted in a dose-dependent decrease in the expression levels of CD47 and cell division control protein 42 (CDC42).
Upregulation of CD47 abolished the role of H2 in promoting lung cancer cell apoptosis and inhibiting cell growth, invasion and migration in A549 and H1975 cell lines. However, CD47 knockdown enhanced the role of H2 in suppressing lung cancer. In addition, we also observed that H2 treatment induced marked suppression of CDC42 and CD47 expression levels in mouse tumor tissues and enhanced macrophage-mediated phagocytosis in A549 and H1975 cells. In conclusion, the current study shows that H2 inhibits lung cancer progression through CD47 downregulation, which may be an effective approach for the treatment of lung cancer.
Research Conclusion
In conclusion, the present study makes clear that H2 exerts an anti-tumor role in lung cancer via down-regulating CD47, an antiphagocytic molecule. As more and more attention has been attracted on the application of H2 therapy in clinical trials [32–34], H2 therapy should be proposed as a promising therapeutic strategy for cancer treatment.
Jinghong Meng; Leyuan Liu; Dongchang Wang; Zhenfeng Yan; Gang Chen
Original PublicationIntroduction
Lung cancer is the most common malignant tumor and the main reason of cancer-related deaths all over the world. It is estimated that the newly diagnosed cases of lung cancer accounted for ∼12.9% among all new cases of tumors in 2012 [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer types, with a 5-year survival rate for patients at stage IV less than 1% [2]. Most patients with NSCLC will develop metastasis when they are first diagnosed [3,4], losing the perfect time for surgery. Therefore, it is essential to find new effective treatment strategies to improve the outcome of NSCLC patients.
Hydrogen gas (H2), as a kind of endogenous gas, has been identified not only to serve as a crucial energy source, but also exerts important physiological regulation roles [5]. Hydrogen molecules can enter into tissues and exert anti-inflammatory, antioxidant and anti-apoptotic roles [6]. As early as 1975, Dole et al. [7] put forward for the first time, that H2 had the ability to treat cancers. In our previous study, we found that H2 administration significantly repressed lung cancer A549 and H1975 cell proliferation, migration and invasion and induced cell apoptosis [8]. However, the molecular mechanisms still remain largely unknown.
CD47 is a transmembrane glycoprotein which is widely expressed in normal tissues and mediates a ‘self/don’t-eat-me’ signaling via repressing macrophage phagocytosis [9,10]. CD47 has been reported to be frequently up-regulated in multiple kinds of cancers, such as ovarian cancer [11], gastric cancer [12], breast cancer [13] and NSCLC [14]. Noticeably, it has been identified that CD47 induces the immunological evasion of cancer cells, and is considered as a potential target for cancer treatment [15,16]. Using sequencing technique, we found that H2 induced a significant reduction in CD47 expression in lung cancer A549 cells, but weather CD47 is involved in H2-mediated inhibition in lung cancer progression still remains unclear.
In the current study, we aimed to elucidate the roles of CD47 in H2-mediated inhibition of lung cancer progression though carrying out both in vivo and in vitro experiments.
Materials and methods
Cell lines and culture
Two lung cancer cell lines A549 and H1975 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, U.S.A.). A549 cells were cultured in F-12K Medium (Gibco, Thermo Fisher Scientific, MA, U.S.A.), supplemented with 10% fetal bovine serum (FBS; Gibco). H1975 cells were grown in RPMI-1640 medium (Gibco) supplemented with 10% FBS. All cell lines were maintained in an incubator at 37°C with 5% CO2.
H2 treatment
A549 and H1975 cells were cultured in 20, 40 and 60% H2 with the help of hydrogen machine (Shanghai Nanobubble Technology Co., Ltd., Shanghai, China) for different times, and 5% CO2 rved as the negative control.
Cell transfection
Vectors used to overexpress CD47 (OE-CD47) and the small interfering RNAs (siRNAs) used to silence CD47 (si-CD47), as well as their negative control vectors (OE-NC, si-NC) were all obtained from GenePharma (Shanghai, China). A549 and H1975 cells were transfected with these vectors using the Lipofectamine 2000 transfected reagent (Invitrogen, Carlsbad, CA, U.S.A.) referring to the manufacturer’s instructions.
Quantitative real-time PCR analysis
The total RNAs were extracted from cells using the RNApure Tissue & Cell Kit (DNase I) in accordance with the manufacturer’s instructions (CWBio, Beijing, China). Then, a total of 1 μg RNA from each sample was subjected to cDNA reverse transcription and real-time PCR (RT-PCR) using the Quant One Step RT-PCR kit (TIANGEN, Beijing, China) on Bio-Rad detection system (Bio-Rad, Hercules, CA) after RNA quantification via using an ND-1000 NanoDrop Spectrophotometer (NanoDrop Technologies, Inc., Wilmington, Delaware). GAPDH expression level serves as an internal reference. Primers were listed as follows,
CD47: forward (F) 5′-CGGCGTGTATACCAATGC-3′; Reverse (R) 5′-TTTGAATGCATTAAGGGGTTCCT-3′;
GAPDH: F 5′-CCACTAGGCGCTCACTGTTCTC-3′; R 5′-ACTCCGACCTTCACCTTCCC-3′.Western blotting assay
Total proteins were extracted from cells using the RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) supplemented with protease inhibitor for 30 min at 4°C. Following quantification with a BCA Protein Kit (Bio- Rad Laboratories, CA, U.S.A.), 30 μg proteins from each sample were separated by 10% polyacrylamide gels, and were transferred to the polyvinylidene difluoride membranes (Millipore, Billerica, MA, U.S.A.). The membranes were then incubated with 5% non-fat milk and probed with the primary antibodies overnight at 4°C, including Bcl-2 (1:1000 dilution; No. #3498, Cell Signaling Technology, MA, U.S.A.), cleaved caspase3 (1:1000 dilution; No. 9661, Cell Signaling Technology), caspase3 (1:1000 dilution; No. #9662, Cell Signaling Technology), cleaved PARP (1:1000 dilution; #5625, Cell Signaling Technology), PARP (1:1000 dilution; #9532, Cell Signaling Technology), CD47 (1:1000 dilution; No. #63000, Cell Signaling Technology), cell division control protein 42 (CDC42) (1:1000 dilution; No. #2462, Cell Signaling Technology), β-actin (1:2000 dilution; No. #4970, Cell Signaling Technology). Following incubation with the corresponding second antibodies (Santa Cruz Biotechnology, Dallas, TX, U.S.A.), the protein expressions were examined on a Western blotting imaging and quantitative system (Bio-Rad) after incubation with chemiluminescent ECL reagent (Millipore). The quantification of proteins was carried out by using the ImageJ software (National Institutes of Health) after background of subtraction, with β-actin expression as an internal reference.
Cell counting kit-8 assay
Cell counting kit-8 (CCK-8) assay was used to detect cell proliferation. In brief, A549 and H1975 cells were seeded in 96-well plates at a density of 3 × 103 cells/well and cultured at 37°C overnight, then the cells were given H2 treatment and/or cell transfection. Following incubation at 37°C for the indicated times, the cell culture medium was replaced with 10 μl of CCK-8 reagent (Beyotime, Beijing, China) and 90 μl fresh medium, and the cells were incubated at 37°C for another 4 h. The absorbance at 450 nm was measured with a plate reader (model 680; Bio-Rad, Hertfordshire, U.K.).
Flow cytometry assay
Flow cytometry assay was carried out for cell apoptosis assessment. In brief, different treated A549 and H1975 cells were harvested and subjected to apoptosis test using Annexin V (FITC)/propidium iodide (PI) Apoptosis Detection Kits (Dojindo, Japan). Cell apoptosis rate was detected by using flow cytometry (Beckman Coulter, CA, U.S.A.) and analyzed using FlowJo 7.6 software (FlowJo LLC, Ashland, OR, U.S.A.).
Wound healing assay
Wound healing assay was used to detect cell migration ability. Briefly, A549 and H1975 cells were plated in six-well plates at a concentration of 5 × 105 cells/ml and incubated at 37°C overnight, followed by different cell transfections. The wounds were made using 20 μl pipette tips when cell confluence reached at 100%, and the gloating cells were removed via PBS washing. Then, the cells were cultured at 37°C with 60% H2 administration or not. Images of cells movement to the scratch area were taken every 6–12 h using a microscope.
Transwell chamber assay
Transwell chambers with 8-μm polycarbonate filters (BD Bioscience, San Diego, CA, U.S.A.) were applied for cell invasion assessment. In procedure, chambers were coated with Matrigel on the lower side. Then, approximately 2 × 105 of A549 or H1975 cells resuspended with FBS-free medium were seeded in the upper chamber, while 600 μl medium supplemented with 20% FBS were added into the lower chamber. Following 48 h of incubation at 37°C with 5% CO2 or 60% H2, cells in the top of the membranes were removed using cotton buds and cells at the bottom of the membrane were fixed and stained with 0.2% Crystal Violet (Solarbio, Beijing, China). The stained cells were counted under a light microscope (magnification: 200×) to assess cell invasiveness.
In vivo experiment
Animal experiment was performed in the Third Hospital of Hebei Medical University by Wang et al. [8] in accordance with National Institute of Health’s Guidelines for the Care and Use of Laboratory Animals, and was given permission by Animal Care and Research Committee of the Third Hospital of Hebei Medical University. Four-week male BALB/c athymic nude mice (Beijing Vital River Laboratory Animal Technology, Beijing, China) were fed in specific pathogen-free conditions. A total of 1 × 107 A549 cells suspended in 200 μl PBS were injected into the BALB/c mice, which were then divided into three groups when the tumors were grown 3–4 mm in diameter, those were H2 group, cis-platinum group and control group. Mice in H2 group were given 60% H2 inhaling for 2 h every day, mice in cis-platinum group were given cis-platinum (10 mg/kg body weight) [17] intraperitoneal injection. H2 and cis-platinum treatments were kept for 4 weeks. Then, the mice were killed via cervical dislocation. Atmosphere was used as a control of 60% H2 and the same amount of saline was served as a control for cis-platinum.
In vitro phagocytosis assay
The ability of macrophage-mediated phagocytosis was measured according to a previous study [18]. In briefly, 1 × 105 macrophages were seeded into the culture dishes with glass bottom. Then, A549 and H1975 cells were labeled with 20 μM CFDA-SE using a Vybrant CFDA-SE Cell Tracer Kit (Invitrogen), which were then inoculated into the macrophages culture. Macrophages were washed and then photographed with confocal microscope. The phagocytic index was evaluated by the number of phagocytosed carboxy fluoresce in succinimidyl ester (CFSE)-positive cells per 100 macrophages.
Hematoxylin and Eosin and immunohistochemistry staining
Mice tissue samples from H2, cis-platinum and control groups were contributed by Wang et al. [8]. The cancer tissues were removed and subjected to Hematoxylin and Eosin (HE) and immunohistochemistry (IHC) staining to evaluate the histopathology and the protein expressions of CD47 and CDC42 referring to the following procedures. In brief, the paraffin-embedded cancer tissues were cut into 4-μm sections, followed by being sectioned, dewaxed and hydrated and incubation with 3% H2O2 for 10 min and antigen retrieval using Tris-EDTA. The sections were then sealed with 5% goat serum and subsequently probed with anti-CD47 (1:200 dilution; No. ab175388, Abcam, Cambridge, MA, U.S.A.) or anti-CDC42 (1:150 dilution; No. ab64533, Abcam) antibody overnight at 4°C and the corresponding secondary antibody. After being washed with PBS for three times, the sections were incubated with chromogen 3,3′-diaminobenzidinetetrachloride (DAB) (Serva, Heidelberg, Germany), which was served as a substrate.
Statistical analysis
Each experiment in the current study was performed in triplicate. Statistical significance comparison between two groups and multiple groups was performed by using Student’s t test and one-way ANOVA, respectively. Data analysis was performed by using GraphPad Prism (version 6.0, La Jolla, CA, U.S.A.). P<0.05 was considered as statistically significant.
Results
H2 administration represses the malignant transformation of lung cancer cells
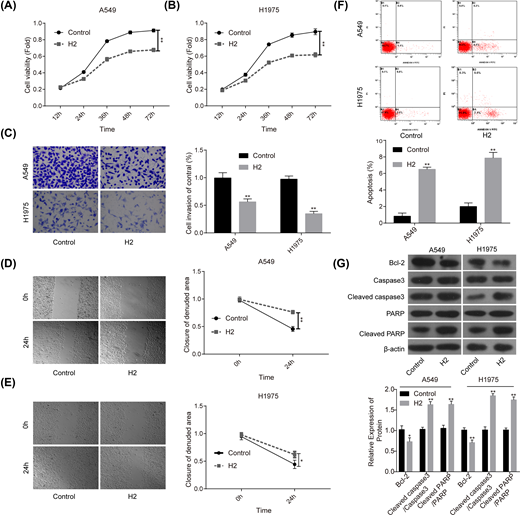
First, we explored the roles of 60% H2 administration in the progression of lung cancer in vitro. Compared with the control group, cell proliferation (Figure 1A,B), invasion (Figure 1C) and migration (Figure 1D,E) abilities were all significantly repressed when cells were given 60% H2 treatment in both A549 and H1975 cell lines. In addition, cell apoptosis rates in both A549 and H1975 cells were obviously increased in H2 groups when compared with that of the control group (Figure 1F), accompanied with decreased expression of Bcl-2 and the increases in cleaved caspase3/caspase3 and cleaved PARP/PARP levels (Figure 1G). These results suggested that H2 treatment could repress the progression of lung cancer in vitro.
H2 treatment inhibited cell proliferation, migration and invasion and induced cell apoptosis in A549 and H1975 cells
Figure 1
H2 treatment inhibited cell proliferation, migration and invasion and induced cell apoptosis in A549 and H1975 cells
CCK-8 assay was used to detect cell proliferation after cells were treated with 60% H2 for 12, 24, 36, 48 and 72 h in (A) A549 and (B) H1975 cells. (C) Cell invasion ability was determined by using the Transwell chambers after 48 h of cell treatment with 60% H2. (D,E) Wound healing assay was used to assess cell migration ability after 24 h of cell treatment with 60% H2. (F) The effects of H2 treatment on cell apoptosis were determined by flow cytometry assay in A549 and H1975 cells. (G) Western blotting was carried out to detect the expressions of Bcl-2, cleaved caspase3, caspase3, cleaved PARP and PARP after 48 h of cell treatment with 60% H2 (*P<0.05, **P<0.01).
Figure 1
H2 treatment inhibited cell proliferation, migration and invasion and induced cell apoptosis in A549 and H1975 cells
CCK-8 assay was used to detect cell proliferation after cells were treated with 60% H2 for 12, 24, 36, 48 and 72 h in (A) A549 and (B) H1975 cells. (C) Cell invasion ability was determined by using the Transwell chambers after 48 h of cell treatment with 60% H2. (D,E) Wound healing assay was used to assess cell migration ability after 24 h of cell treatment with 60% H2. (F) The effects of H2 treatment on cell apoptosis were determined by flow cytometry assay in A549 and H1975 cells. (G) Western blotting was carried out to detect the expressions of Bcl-2, cleaved caspase3, caspase3, cleaved PARP and PARP after 48 h of cell treatment with 60% H2 (*P<0.05, **P<0.01).H2 treatment down-regulates the expression of CD47 and CDC42 in lung cancer
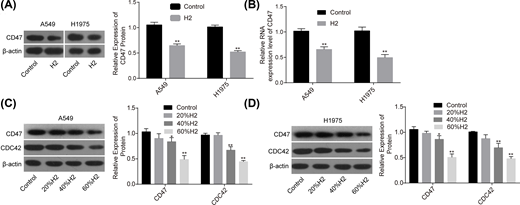
Our research group previously revealed that H2 treatment could induce a significant decrease in CD47 expression [8], indicating that CD47 might be involved in H2-mediated lung cancer inhibition. To this end, we used Western blotting assay to explore the effect of H2 treatment on the expression of CD47. Compared with the control group, the mRNA and protein expression levels of CD47 were all significantly decreased when A549 and H1975 cells were treated with 60% H2 (Figure 2A,B). Moreover, H2 treatment decreased CD47 and CDC42 expressions in a dose-dependent manner in A549 (Figure 2C) and H1975 cells (Figure 2D). These results demonstrated that H2 negatively regulated CD47 and CDC42 expression in lung cancer.
H2 treatment decreased the expressions of CD47 and CDC42 in A549 and H1975 cells
Figure 2
H2 treatment decreased the expressions of CD47 and CDC42 in A549 and H1975 cells
After 48 h of cell treatment with H2, A549 and H1975 cells were collected and subjected to RNA and DNA extraction with the following detections. (A,B) The mRNA and protein levels of CD47 were detected by RT-PCR and Western blotting assays. (C,D) The protein expression levels of CD47 and CDC42 were detected by Western blotting after cell treatments with different concentrations of H2 (*P<0.05, **P<0.01).Figure 2
H2 treatment decreased the expressions of CD47 and CDC42 in A549 and H1975 cells
After 48 h of cell treatment with H2, A549 and H1975 cells were collected and subjected to RNA and DNA extraction with the following detections. (A,B) The mRNA and protein levels of CD47 were detected by RT-PCR and Western blotting assays. (C,D) The protein expression levels of CD47 and CDC42 were detected by Western blotting after cell treatments with different concentrations of H2 (*P<0.05, **P<0.01).Knockdown of CD47 cooperates with H2 to repress the progression of lung cancer
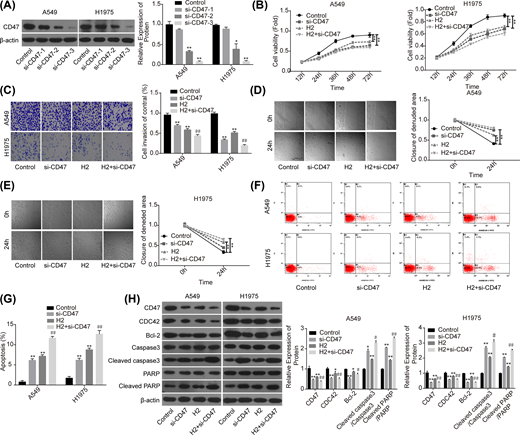
Then, we performed the loss-of-function assay to explore the role of CD47 in H2-mediated lung cancer repression. Compared with the si-NC group, the expression of CD47 was significantly decreased when A549 and H1975 cells were transfected with si-CD47, and si-CD47-3 showed the best knockdown efficiency (Figure 3A). Compared with the control group, cell proliferation (Figure 3B), invasion (Figure 3C) and migration (Figure 3D,E) capacities were all significantly decreased when the cells were transfected with si-CD47 or treated with 60% H2. In addition, both down-regulation of CD47 and H2 treatment induced a significant increase in cell apoptosis rate (Figure 3F,G), and decreases in the expressions of Bcl-2 and CDC42, as well as the increasein the levels of cleaved caspase3/caspase3 and cleaved PARP/PARP (Figure 3H). Noticeably, combination of si-CD47 with H2 treatment showed higher efficiency than si-CD47 or H2 treatment alone (Figure 3B–H). These findings illustrated that CD47 down-regulation could enhance H2 effect on the repression of lung cancer progression.
Knockdown of CD47 cooperated with H2 to repress the progression of lung cancer
Figure 3
Knockdown of CD47 cooperated with H2 to repress the progression of lung cancer
A549 and H1975 cells transfected with si-CD47 or si-NC were treated with 60% H2, then the following assays were performed. (A) Western blotting assay was used to detect the knockdown efficiency of si-CD47 in A549 and H1975 cells. (B) CCK-8 assay was used to detect cell proliferation. (C) Transwell chambers were applied for cell invasion assessment. (D,E) Wound healing assay was used to detect cell migration ability. (F,G) The effects of H2 treatment on the apoptosis of A549 and H1975 cells were determined by flow cytometry assay. (H) Western blotting was carried out to detect the expressions of CD47, CDC42, Bcl-2, cleaved caspase3, caspase3, cleaved PARP and PARP after 48 h of cell treatment with 60% H2 (*P<0.05, **P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. H2 group).
Figure 3
Knockdown of CD47 cooperated with H2 to repress the progression of lung cancer
A549 and H1975 cells transfected with si-CD47 or si-NC were treated with 60% H2, then the following assays were performed. (A) Western blotting assay was used to detect the knockdown efficiency of si-CD47 in A549 and H1975 cells. (B) CCK-8 assay was used to detect cell proliferation. (C) Transwell chambers were applied for cell invasion assessment. (D,E) Wound healing assay was used to detect cell migration ability. (F,G) The effects of H2 treatment on the apoptosis of A549 and H1975 cells were determined by flow cytometry assay. (H) Western blotting was carried out to detect the expressions of CD47, CDC42, Bcl-2, cleaved caspase3, caspase3, cleaved PARP and PARP after 48 h of cell treatment with 60% H2 (*P<0.05, **P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. H2 group).
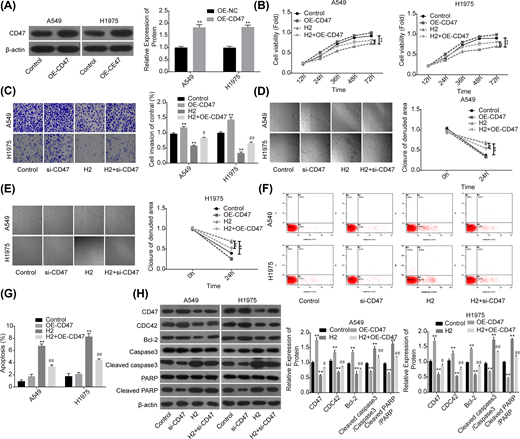
Overexpression of CD47 impairs H2 role in repressing the progression of lung cancer
To further reveal the role of CD47 in H2-mediated lung cancer inhibition, we also performed the gain-of-function assay. The expression of CD47 was apparently increased when A549 and H1975 cells were transfected with OE-CD47 (Figure 4A). To the opposite of H2 roles in lung cancer functions alteration, cell proliferation (Figure 4B), invasion (Figure 4C) and migration (Figure 4D,E) abilities were all obviously enhanced when cells were transfected with OE-CD47. Moreover, CD47 overexpression significantly inhibited cell apoptosis (Figure 4F,G) and increased the expression of Bcl-2 while decreased the expression levels of cleaved caspase3/caspase3 and cleaved PARP/PARP (Figure 4H). Furthermore, CD47 overexpression significantly abolished H2 roles in the inhibitions of cell growth, migration, invasion and Bcl-2 expression, as well as the promotions of cell apoptosis and the expressions of cleaved caspase3/caspase3 and cleaved PARP/PARP (Figure 4B–H). These results further confirmed that H2 treatment inhibited the progression of lung cancer via decreasing CD47 expression.
Overexpression of CD47 impaired H2 roles in repressing the progression of lung cancer
Figure 4
Overexpression of CD47 impaired H2 roles in repressing the progression of lung cancer
A549 and H1975 cells transfected with OE-CD47 or OE-NC were treated with 60% H2, then the following assays were performed. (A) Western blotting assay was used to detect the protein expression of CD47 after A549 and H1975 cells were transfected with OE-CD47 or OE-NC. (B) CCK-8 assay was used to detect cell proliferation. (C) Transwell chambers were applied for cell invasion assessment. (D,E) Wound healing assay was used to detect cell migration ability. (F,G) The effects of H2 treatment on the apoptosis of A549 and H1975 cells were determined by flow cytometry assay. (H) Western blotting was carried out to detect the expressions of CD47, CDC42, Bcl-2, cleaved caspase3, caspase3, cleaved PARP and PARP after 48 h of cell treatment with 60% H2 (*P<0.05, **P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. H2 group).
Figure 4
Overexpression of CD47 impaired H2 roles in repressing the progression of lung cancer
A549 and H1975 cells transfected with OE-CD47 or OE-NC were treated with 60% H2, then the following assays were performed. (A) Western blotting assay was used to detect the protein expression of CD47 after A549 and H1975 cells were transfected with OE-CD47 or OE-NC. (B) CCK-8 assay was used to detect cell proliferation. (C) Transwell chambers were applied for cell invasion assessment. (D,E) Wound healing assay was used to detect cell migration ability. (F,G) The effects of H2 treatment on the apoptosis of A549 and H1975 cells were determined by flow cytometry assay. (H) Western blotting was carried out to detect the expressions of CD47, CDC42, Bcl-2, cleaved caspase3, caspase3, cleaved PARP and PARP after 48 h of cell treatment with 60% H2 (*P<0.05, **P<0.01 vs. control group; #P<0.05, ##P<0.01 vs. H2 group).
H2 treatment represses the expressions of CD47 and CDC42 in lung cancer tissues from in vivo mice
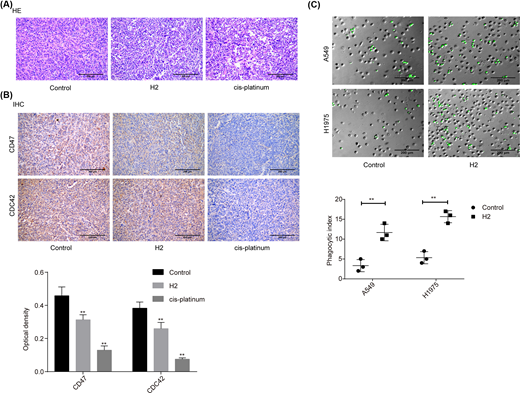
In addition, we investigated the role of H2 in lung cancer progression in vivo using the cancer tissues samples from our previous study [8]. As shown in the HE image, tumor cells were closely packed and cell atypia was obvious in the tumor tissues of the control group. Compared with the control group, tumor cell density and cell atypia was obviously decreased in both H2 and cis-platinum groups (Figure 5A). In addition, the expression levels of CD47 and CDC42 were decreased in both H2 and cis-platinum groups compared with the control group (Figure 5B). As CD47 is a regulator of macrophage-mediated phagocytosis [18], we conjectured that H2 might play a role in macrophage-mediated phagocytosis. Consistent to our assumption, we observed that the phagocytic index was significantly increased when A549 cells were administrated with 60% H2 in both A549 and H1975 cell lines (Figure 5C). These results further confirmed that H2 treatment repressed the progression of lung cancer via modulating CD47 expression.
H2 treatment repressed the progression of lung cancer in vivo
Figure 5
H2 treatment repressed the progression of lung cancer in vivo
(A) HE staining was carried out to assess the histopathology of lung cancer tissues from different treated mice (scale bar = 200 μm). (B) IHC staining was used to evaluate the expressions of CD47 and CDC42 in lung cancer tissues from different treated mice (scale bar = 200 μm). (C) In vitro phagocytosis assay was used to assess the effect of H2 treatment on macrophage-mediated phagocytosis in A549 and H1975 cells (scale bar = 200 μm) (**P<0.01).
Figure 5
H2 treatment repressed the progression of lung cancer in vivo
(A) HE staining was carried out to assess the histopathology of lung cancer tissues from different treated mice (scale bar = 200 μm). (B) IHC staining was used to evaluate the expressions of CD47 and CDC42 in lung cancer tissues from different treated mice (scale bar = 200 μm). (C) In vitro phagocytosis assay was used to assess the effect of H2 treatment on macrophage-mediated phagocytosis in A549 and H1975 cells (scale bar = 200 μm) (**P<0.01).
Discussion
Hydrogen presents mild reductive productivity with no toxicity in itself, which is beneficial to prevent and control serious toxicity side effects in medical procedures [19]. Since Dole et al. [7] put forward that H2 had the ability to treat cancers in 1975, Ohsawa et al. [6] found that Hydrogen had an anti-oxidant role which could selectively eliminate reactive oxygen species (ROS) in 2007. As to cancer, both increase and decrease in ROS levels all can break the redox homeostasis and bring redox stress, leading to cancer cell damage [20,21]. This discovery suggests that hydrogen exerts its anti-tumor role might via altering intratumoural ROS level. In addition, our research group found that H2 repressed the progression of lung cancer via regulating the expression of structural maintenance of chromosomes 3 (SMC3), which is an important regulator of chromosome condensation [8]. With the aim of further exploration of the mechanism of H2 in suppressing lung cancer progression, the present study illustrates that H2 represses the progression of lung cancer via down-regulating CD47.
In the current study, we observed that H2 treatment significantly decreased the expression levels of CD47 and CDC42. CD47, also termed as integrin-associated protein, is a 50-kDa cell surface glycoprotein and belongs to the immunoglobulin superfamily. CD47 is widely expressed on cell surface and serves as an antiphagocytic molecule via binding to signal regulatory protein α (SIRPα) [22,23]. CDC42 is a member of the Rho family of small GTPases and is activated by CD47 to serve as a crucial regulator of cancer metastasis [24]. Increasing evidences have shown that CD47 expression was elevated in tumor cancer tissues and cells, such as bladder cancer [25], leukemia [26], colon cancer [27] and NSCLC [28]. Its overexpression closely correlated with patients’ poor prognosis and significantly accelerated tumor cell transformation to malignant phenotypes. For example, Majeti et al. [26] reported that CD47 was highly expressed in acute myeloid leukemia (AML), and its increased expression level predicted shorter overall survival in adult AML patients. Moreover, blockage of CD47 with monoclonal antibodies enabled macrophage phagocytosis of AML leukemia stem cells and inhibited their tumorigenesis in vivo. Inhibition of CD47 with blocking antibodies or siRNA transfection obviously inhibited the migration of colon cancer SW480 cells in the presence of M2 macrophages [27]. Yoshida et al. [29] reported that CD47 was positively expressed in 49.6% (57/115) gastric cancer tissues, and its expression levels closely associated with the poor prognosis in gastric cancer. Taken together, CD47 is identified as a commonly expressed molecule on cancers, and blockade of its function leads to tumor cell phagocytosis and elimination. Therefore, CD47 is a proposed target for cancer therapies [30]. Here, we revealed that H2 treatment could negatively modulate CD47 expression, which might be the mechanism by which H2 inhibited lung cancer progression.
To further reveal CD47 roles in H2-mediated repression of lung cancer progression, we carried out the gain/loss-of-function assays. We observed that down-regulation of CD47 with si-CD47 transfection significantly enhanced H2 effects on the repressions of cell proliferation, invasion and migration, and the promotion of cell apoptosis. However, these above roles of H2 were apparently weakened when CD47 was overexpressed. These results indicate that H2 inhibits the progression of lung cancer via down-regulating CD47.
Zhang et al. [31] reported that blocking CD47 with a novel CD47-targeting fusion protein SIRPaD1-Fc led to a significant increase in macrophage-mediated phagocytosis in NSCLC cells, further confirming the antiphagocytic role of CD47 in lung cancer [28]. Based on this, we assume that H2 could also exert an antiphagocytic role in lung cancer cells. Consistently, we found that macrophage-mediated phagocytosis was significantly enhanced when cells were treated with H2 in both A549 and H1975 cells. Moreover, H2 administration could significantly alleviate the pathological change of lung cancer tissues from the in vivo mice, as well as cause obvious decreases in CD47 and CDC42 expressions.
In conclusion, the present study makes clear that H2 exerts an anti-tumor role in lung cancer via down-regulating CD47, an antiphagocytic molecule. As more and more attention has been attracted on the application of H2 therapy in clinical trials [32–34], H2 therapy should be proposed as a promising therapeutic strategy for cancer treatment.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Program of Studying the Molecular Mechanism and Clinical Transformation of Hydrogen in the Treatment of Lung Cancer [grant number zh2018006].
Author Contribution
Gang Chen provided the idea of the present study and revised the manuscript. Jinghong Meng did most of the experiments and wrote the manuscript. Leyuan Liu did parts of the experiments and the data analysis. Dongchang Wang did the animal experiment and provided the animal tissues. Zhenfeng Yan did parts of the experiments.
Abbreviations
AML – acute myeloid leukemia
CCK-8 – cell counting kit-8
CDC42 – cell division control protein 42
FBS – fetal bovine serum
HE – Hematoxylin and Eosin
H2 – hydrogen gas
PARP – poly ADP-ribose polymerase
ROS – reactive oxygen species
RT-PCR – real-time PCR
siRNA – small interfering RNA
1. (2017) All cancers estimated incidence, mortality and prevalence of all cancers (excluding non-melanoma skin cancer) in 2012. J. Natl. Cancer Inst. 109, https://doi.org/10.1093/jnci/djx205
2. Travis W.D.
(2011) Pathology of lung cancer. Clin. Chest Med. 32, 669–692 https://doi.org/10.1016/j.ccm.2011.08.005
3. Morgensztern D., Ng S.H., Gao F. and Govindan R.
(2010) Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J. Thorac. Oncol. 5, 29–33 https://doi.org/10.1097/JTO.0b013e3181c5920c
4. Carney D.N.and Hansen H.H.
(2000) Non-small-cell lung cancer–stalemate or progress? N. Engl. J. Med. 343, 1261-1262 https://doi.org/10.1056/NEJM200010263431710
5. Zhao P., Jin Z., Chen Q., Yang T., Chen D., Meng J. et al.
(2018) Local generation of hydrogen for enhanced photothermal therapy. Nat. Commun. 9, 4241 https://doi.org/10.1038/s41467-018-06630-2
6. Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K. et al.
(2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13, 688- 694 https://doi.org/10.1038/nm1577
7. Dole M., Wilson F.R. and Fife W.P.
(1975) Hyperbaric hydrogen therapy: a possible treatment for cancer. Science 190, 152-154 https://doi.org/10.1126/science.1166304
8. Wang D., Wang L., Zhang Y., Zhao Y. and Chen G.
(2018) Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed. Pharmacother. 104, 788-797 https://doi.org/10.1016/j.biopha.2018.05.055
9. Baccelli I., Schneeweiss A., Riethdorf S., Stenzinger A., Schillert A., Vogel V. et al.
(2013) Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544 https://doi.org/10.1038/nbt.2576
10. Tsai R.K. and Discher D.E.
(2008) Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J. Cell Biol. 180, 989–1003 https://doi.org/10.1083/jcb.200708043
11. Li Y., Lu S., Xu Y., Qiu C., Jin C., Wang Y. et al.
(2017) Overexpression of CD47 predicts poor prognosis and promotes cancer cell invasion in high-grade serous ovarian carcinoma. Am. J. Transl. Res. 9, 2901–2910
12. Sudo T., Takahashi Y., Sawada G., Uchi R., Mimori K. and Akagi Y.
(2017) Significance of CD47 expression in gastric cancer. Oncol. Lett. 14, 801–809 https://doi.org/10.3892/ol.2017.6257
13. Baccelli I., Stenzinger A., Vogel V., Pfitzner B.M., Klein C., Wallwiener M. et al.
(2014) Co-expression of MET and CD47 is a novel prognosticator for survival of luminal breast cancer patients. Oncotarget 5, 8147–8160 https://doi.org/10.18632/oncotarget.2385
14. Barrera L., Montes-Servin E., Hernandez-Martinez J.M., Garcia-Vicente M.L.A., Herrera-Martinez M., Crispin J.C. et al.
(2017) CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br. J. Cancer 117, 385–397 https://doi.org/10.1038/bjc.2017.173
15. Rodriguez P.L., Harada T., Christian D.A., Pantano D.A., Tsai R.K. and Discher D.E.
(2013) Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science 339, 971–975 https://doi.org/10.1126/science.1229568
16. Kong F., Gao F., Li H., Liu H., Zhang Y., Zheng R. et al.
(2016) CD47: a potential immunotherapy target for eliminating cancer cells. Clin. Transl. Oncol. 18, 1051–1055 https://doi.org/10.1007/s12094-016-1489-x
17. Yang W., Yang X., Wang X., Gu J., Zhou D., Wang Y. et al.
(2019) Silencing CDR1as enhances the sensitivity of breast cancer cells to drug resistance by acting as a miR-7 sponge to down-regulate REGgamma. J. Cell. Mol. Med. https://doi.org/10.1111/jcmm.14305
18. Cabrales P.
(2019) RRx-001 acts as a dual small molecule checkpoint inhibitor by downregulating CD47 on cancer cells and SIRP-alpha on monocytes/macrophages. Transl. Oncol. 12, 626–632 https://doi.org/10.1016/j.tranon.2018.12.001
19. Cui J., Chen X., Zhai X., Shi D., Zhang R., Zhi X. et al.
(2016) Inhalation of water electrolysis-derived hydrogen ameliorates cerebral ischemia-reperfusion injury in rats – a possible new hydrogen resource for clinical use. Neuroscience 335, 232–241 https://doi.org/10.1016/j.neuroscience.2016.08.021
20. Trachootham D., Alexandre J. and Huang P.
(2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 https://doi.org/10.1038/nrd2803
21. Kim J. and Bae J.S.
(2016) ROS homeostasis and metabolism: a critical liaison for cancer therapy. Exp. Mol. Med. 48, e269 https://doi.org/10.1038/emm.2016.119
22. Matozaki T., Murata Y., Okazawa H. and Ohnishi H.
(2009) Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 19, 72–80 https://doi.org/10.1016/j.tcb.2008.12.001
23. Ide K., Wang H., Tahara H., Liu J., Wang X., Asahara T. et al.
(2007) Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc. Natl. Acad. Sci. U.S.A. 104, 5062–5066 https://doi.org/10.1073/pnas.0609661104
24. Chang J.S., Su C.Y., Yu W.H., Lee W.J., Liu Y.P., Lai T.C. et al.
(2015) GIT1 promotes lung cancer cell metastasis through modulating Rac1/Cdc42 activity and is associated with poor prognosis. Oncotarget 6, 36278–36291 https://doi.org/10.18632/oncotarget.5531
25. Chan K.S., Espinosa I., Chao M., Wong D., Ailles L., Diehn M. et al.
(2009) Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. U.S.A. 106, 14016–14021 https://doi.org/10.1073/pnas.0906549106
26. Majeti R., Chao M.P., Alizadeh A.A., Pang W.W., Jaiswal S., Gibbs K.D.Jr et al.
(2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, 286–299 https://doi.org/10.1016/j.cell.2009.05.045
27. Zhang Y., Sime W., Juhas M. and Sjolander A.
(2013) Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur. J. Cancer 49, 3320–3334 https://doi.org/10.1016/j.ejca.2013.06.005
28. Zhao H., Wang J., Kong X., Li E., Liu Y., Du X. et al.
(2016) CD47 promotes tumor invasion and metastasis in non-small cell lung cancer. Sci. Rep. 6, 29719 https://doi.org/10.1038/srep29719
29. Yoshida K., Tsujimoto H., Matsumura K., Kinoshita M., Takahata R., Matsumoto Y. et al.
(2015) CD47 is an adverse prognostic factor and a therapeutic target in gastric cancer. Cancer Med. 4, 1322–1333 https://doi.org/10.1002/cam4.478
30. Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S. et al.
(2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. U.S.A. 109, 6662–6667 https://doi.org/10.1073/pnas.1121623109
31. Zhang X., Fan J., Wang S., Li Y., Wang Y., Li S. et al.
(2017) Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol. Res. 5, 363–375 https://doi.org/10.1158/2326-6066.CIR-16-0398
32. Nagatani K., Nawashiro H., Takeuchi S., Tomura S., Otani N., Osada H. et al.
(2013) Safety of intravenous administration of hydrogen-enriched fluid in patients with acute cerebral ischemia: initial clinical studies. Med. Gas. Res. 3, 13 https://doi.org/10.1186/2045-9912-3-13
33. Yoritaka A., Takanashi M., Hirayama M., Nakahara T., Ohta S. and Hattori N.
(2013) Pilot study of H(2) therapy in Parkinson’s disease: a randomized double-blind placebo-controlled trial. Mov. Disord. 28, 836–839 https://doi.org/10.1002/mds.25375
34. Tamura T., Hayashida K., Sano M., Suzuki M., Shibusawa T., Yoshizawa J. et al.
(2016) Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome-first-in-human pilot study. Circ. J. 80, 1870–1873 https://doi.org/10.1253/circj.CJ-16-0127
© 2020 The Author(s).
2020 This is an open access article published by Portland Press Limited on behalf of the Biochemical Society and distributed under the Creative Commons Attribution License 4.0 (CC BY).
Content retrieved from: https://portlandpress.com/bioscirep/article/40/4/BSR20192761/222726/Hydrogen-gas-represses-the-progression-of-lung.