Hydrogen inhalation, patients with Parkinson’s diseaseScientific Research
Inhalation of hydrogen gas elevates urinary 8-hydroxy-2′-deoxyguanine in Parkinson’s disease
Abstract
Hypoemia is one of the earliest and most common symptoms in Parkinson’s disease (PD). The benefits of hydrogen water on motor deficits have been reported in PD animal models and PD patients, but the effects of hydrogen on PD patients have not been studied. We conducted an 8-week washout study in 20 PD patients in a randomized, double-blind, placebo-controlled, crossover study. Patients inhaled approximately 1.2-1.4% hydrogen-air mixture or placebo for 10 minutes twice a day for 4 weeks. Low-dose hydrogen inhalation did not significantly affect clinical PD parameters, but increased urinary 8-OHdG concentrations by 16%. This increase in 8-OHdG was significantly lower than the over 300% increase in diabetes and more comparable to the increase after vigorous exercise. Although elevated reactive oxygen species are often associated with toxicity and disease, they also play an important role in mediating cytoprotective cellular adaptations in a process known as excitability. Increased hydrogen-induced oxidative stress and its ability to activate Nrf2, the NF-κB pathway, and the heat shock response have been previously reported. Although we did not observe any beneficial effects of hydrogen in our brief experiments, we believe that increased 8-OHdG and other reported hydrogen stress responses may suggest that its beneficial effects are partly or largely mediated by excitatory mechanisms. This study was approved by the Ethics Committee of the Nagoya University Graduate School of Medicine (approval number 2015-0295). This clinical study is registered with the University Hospital’s Medical Information Network (ID UMIN000019082).
Results
A randomized, double-blind, placebo-controlled, crossover study of 20 Parkinson’s disease patients inhaling approximately 1.2-1.4% hydrogen-air mixture or placebo for 10 minutes twice a day for 4 weeks found that OSIT- J (p = 0.77), UPDSR1 (P = 0.84) and UPDRS2 (P = 0.15) were not affected by hydrogen (Table 1). In contrast, 4 weeks of hydrogen inhalation increased urinary 8-OHdG excretion by 16%, which was statistically significant (P=0.02) (Table 1 and Figure 2).
Table 1
Metrics before and after inhalation of true and placebo hydrogen gas for 4 weeks
| Hydrogen | Placebo | |||||
|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | |
| OSIT-J | 4.7±2.0 | 4.85±1.9 | 0.77 | 4.55±2.4 | 4.6±2.2 | 0.92 |
| UPDRS1 | 2.2±1.6 | 2.3±1.9 | 0.84 | 2.7±2.4 | 2.4±1.9 | 0.41 |
| UPDRS2 | 13.7±9.0 | 15.9±7.0 | 0.15 | 16.6±9.1 | 16.8±6.3 | 0.93 |
| 8-OHdG/Cr (ng/mg Cr) | 9.5±9.7 | 11.0±5.9 | 0.02 | 9.2±6.9 | 9.7±6.8 | 0.59 |
Note: Date are expressed as the mean ± SD. P values are calculated by paired t-test. 8-OHdG: 8-Hydroxy-2′-deoxyguanine; Cr: creatine; UPDRS: Unified Parkinson’s Disease Rating Scale.
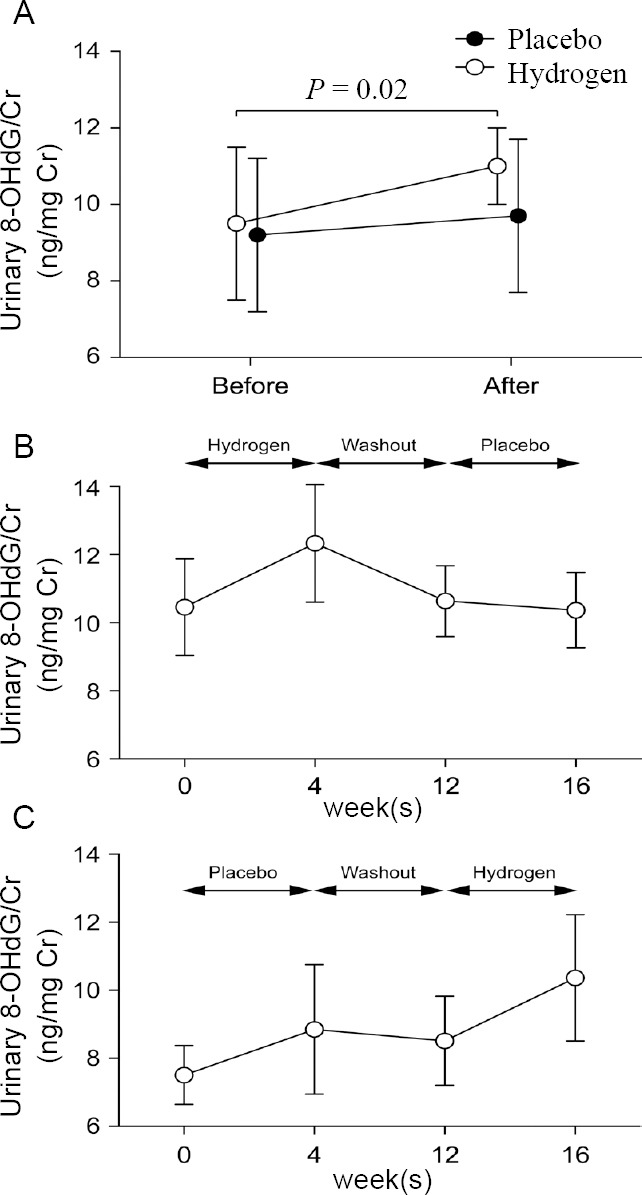
Urinary 8-OHdG/Cr before and after inhalation of true and placebo hydrogen gas for 4 weeks.
Note: (A) Twenty Parkinson’s disease (PD) patients. P value by Student’s paired t-test is indicated. (B) Ten PD patients who inhaled hydrogen first. (C) Ten PD patients who inhaled placebo first. (B, C) No statistical significance by one-way analysis of variance. Date are expressed as the mean ± SE. 8-OHdG: 8-Hydroxy- 2′-deoxyguanine; Cr: creatine.
References
Published on: 20180812
Inhalation of hydrogen gas elevates urinary 8-hydroxy-2′-deoxyguanine in Parkinson’s disease
Abstract
Hypoemia is one of the earliest and most common symptoms in Parkinson’s disease (PD). The benefits of hydrogen water on motor deficits have been reported in PD animal models and PD patients, but the effects of hydrogen on PD patients have not been studied. We conducted an 8-week washout study in 20 PD patients in a randomized, double-blind, placebo-controlled, crossover study. Patients inhaled approximately 1.2-1.4% hydrogen-air mixture or placebo for 10 minutes twice a day for 4 weeks. Low-dose hydrogen inhalation did not significantly affect clinical PD parameters, but increased urinary 8-OHdG concentrations by 16%. This increase in 8-OHdG was significantly lower than the over 300% increase in diabetes and more comparable to the increase after vigorous exercise. Although elevated reactive oxygen species are often associated with toxicity and disease, they also play an important role in mediating cytoprotective cellular adaptations in a process known as excitability. Increased hydrogen-induced oxidative stress and its ability to activate Nrf2, the NF-κB pathway, and the heat shock response have been previously reported. Although we did not observe any beneficial effects of hydrogen in our brief experiments, we believe that increased 8-OHdG and other reported hydrogen stress responses may suggest that its beneficial effects are partly or largely mediated by excitatory mechanisms. This study was approved by the Ethics Committee of the Nagoya University Graduate School of Medicine (approval number 2015-0295). This clinical study is registered with the University Hospital’s Medical Information Network (ID UMIN000019082).
Results
A randomized, double-blind, placebo-controlled, crossover study of 20 Parkinson’s disease patients inhaling approximately 1.2-1.4% hydrogen-air mixture or placebo for 10 minutes twice a day for 4 weeks found that OSIT- J (p = 0.77), UPDSR1 (P = 0.84) and UPDRS2 (P = 0.15) were not affected by hydrogen (Table 1). In contrast, 4 weeks of hydrogen inhalation increased urinary 8-OHdG excretion by 16%, which was statistically significant (P=0.02) (Table 1 and Figure 2).
Table 1
Metrics before and after inhalation of true and placebo hydrogen gas for 4 weeks
Hydrogen Placebo Before After P Before After P OSIT-J 4.7±2.0 4.85±1.9 0.77 4.55±2.4 4.6±2.2 0.92 UPDRS1 2.2±1.6 2.3±1.9 0.84 2.7±2.4 2.4±1.9 0.41 UPDRS2 13.7±9.0 15.9±7.0 0.15 16.6±9.1 16.8±6.3 0.93 8-OHdG/Cr (ng/mg Cr) 9.5±9.7 11.0±5.9 0.02 9.2±6.9 9.7±6.8 0.59 Note: Date are expressed as the mean ± SD. P values are calculated by paired t-test. 8-OHdG: 8-Hydroxy-2′-deoxyguanine; Cr: creatine; UPDRS: Unified Parkinson’s Disease Rating Scale.
Urinary 8-OHdG/Cr before and after inhalation of true and placebo hydrogen gas for 4 weeks.
Note: (A) Twenty Parkinson’s disease (PD) patients. P value by Student’s paired t-test is indicated. (B) Ten PD patients who inhaled hydrogen first. (C) Ten PD patients who inhaled placebo first. (B, C) No statistical significance by one-way analysis of variance. Date are expressed as the mean ± SE. 8-OHdG: 8-Hydroxy- 2′-deoxyguanine; Cr: creatine.
References
1. Ansari KA, Johnson A. Olfactory function in patients with Parkinson’s disease. J Chronic Dis. 1975;28:493–497. [PubMed] [Google Scholar]2. Doty RL, Singh A, Tetrud J, Langston JW. Lack of major olfactory dysfunction in MPTP-induced parkinsonism. Ann Neurol. 1992;32:97–100. [PubMed] [Google Scholar]3. Tissingh G, Berendse HW, Bergmans P, et al. Loss of olfaction in de novo and treated Parkinson’s disease: possible implications for early diagnosis. Mov Disord. 2001;16:41–46. [PubMed] [Google Scholar]4. Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:509. [PubMed] [Google Scholar]5. Baba T, Takeda A, Kikuchi A, et al. Association of olfactory dysfunction and brain. Metabolism in Parkinson’s disease. Mov Disord. 2011;26:621–628. [PubMed] [Google Scholar]6. Lerner A, Bagic A. Olfactory pathogenesis of idiopathic Parkinson disease revisited. Mov Disord. 2008;23:1076–1084. [PubMed] [Google Scholar]7. Duda JE, Shah U, Arnold SE, Lee VM, Trojanowski JQ. The expression of alpha-, beta-, and gamma-synucleins in olfactory mucosa from patients with and without neurodegenerative diseases. Exp Neurol. 1999;160:515–522. [PubMed] [Google Scholar]8. Tofaris GK, Garcia Reitbock P, Humby T, et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. J Neurosci. 2006;26:3942–3950. [PMC free article] [PubMed] [Google Scholar]9. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. [PubMed] [Google Scholar]10. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. [PubMed] [Google Scholar]11. Saito S, Ayabe-Kanamura S, Takashima Y, et al. Development of a smell identification test using a novel stick-type odor presentation kit. Chem Senses. 2006;31:379–391. [PubMed] [Google Scholar]12. Iijima M, Kobayakawa T, Saito S, et al. Smell identification in Japanese Parkinson’s disease patients: using the odor stick identification test for Japanese subjects. Intern Med. 2008;47:1887–1892. [PubMed] [Google Scholar]13. Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249:1–5. [PubMed] [Google Scholar]14. Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. 2013;3:461–491. [PMC free article] [PubMed] [Google Scholar]15. Sato S, Mizuno Y, Hattori N. Urinary 8-hydroxydeoxyguanosine levels as a biomarker for progression of Parkinson disease. Neurology. 2005;64:1081–1083. [PubMed] [Google Scholar]16. Hirayama M, Nakamura T, Watanabe H, et al. Urinary 8-hydroxydeoxyguanosine correlate with hallucinations rather than motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:46–49. [PubMed] [Google Scholar]17. Ilida A, Nosaka N, Yumoto T, et al. The clinical application of hydrogen as a medical treatment. Acta Med Okayama. 2016;70:331–337. [PubMed] [Google Scholar]18. Fu Y, Ito M, Fujita Y, et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci Lett. 2009;453:81–85. [PubMed] [Google Scholar]19. Fujita K, Seike T, Yutsudo N, et al. Hydrogen in drinking water reduces dopaminergic neuronal loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. PLoS One. 2009;4:e7247. [PMC free article] [PubMed] [Google Scholar]20. Ito M, Hirayama M, Yamai K, et al. Drinking hydrogen water and intermittent hydrogen gas exposure, but not lactulose or continuous hydrogen gas exposure, prevent 6-hydorxydopamine-induced Parkinson’s disease in rats. Med Gas Res. 2012;2:15. [PMC free article] [PubMed] [Google Scholar]21. Yoritaka A, Takanashi M, Hirayama M, Nakahara T, Ohta S, Hattori N. Pilot study of H2 therapy in Parkinson’s disease: a randomized double-blind placebo-controlled trial. Mov Disord. 2013;28:836–839. [PubMed] [Google Scholar]22. Katsumata Y, Sano F, Abe T, et al. The effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infarction-first pilot study in humans. Circ J. 2017;81:940–947. [PubMed] [Google Scholar]23. Ono H, Nishijima Y, Ohta S, et al. Hydrogen gas inhalation treatment in acute cerebral infarction: a randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis. 2017;26:2587–2594. [PubMed] [Google Scholar]24. Venetsanos AG, Huld T, Adams P, Bartzis JG. Source, dispersion and combustion modelling of an accidental release of hydrogen in an urban environment. J Hazard Mater. 2003;105:1–25. [PubMed] [Google Scholar]25. Sobue S, Yamai K, Ito M, et al. Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol Cell Biochem. 2015;403:231–241. [PubMed] [Google Scholar]26. Ishibashi T, Sato B, Rikitake M, et al. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med Gas Res. 2012;2:27. [PMC free article] [PubMed] [Google Scholar]27. Yamaguchi Y, Haginaka J, Morimoto S, Fujioka Y, Kunitomo M. Facilitated nitration and oxidation of LDL in cigarette smokers. Eur J Clin Invest. 2005;35:186–193. [PubMed] [Google Scholar]28. Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. [PubMed] [Google Scholar]29. Dandona P, Thusu K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:444–445. [PubMed] [Google Scholar]30. Okamura K, Doi T, Hamada K, et al. Effect of repeated exercise on urinary 8-hydroxy-deoxyguanosine excretion in humans. Free Radic Res. 1997;26:507–514. [PubMed] [Google Scholar]31. Orhan H, van Holland B, Krab B, et al. Evaluation of a multi-parameter biomarker set for oxidative damage in man: increased urinary excretion of lipid, protein and DNA oxidation products after one hour of exercise. Free Radic Res. 2004;38:1269–1279. [PubMed] [Google Scholar]32. Gomes EC, Silva AN, de Oliveira MR. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid Med Cell Longev. 2012;2012:756132. [PMC free article] [PubMed] [Google Scholar]33. Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007;103:1093–1098. [PubMed] [Google Scholar]34. Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. [PubMed] [Google Scholar]35. Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical view of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•OH–) in aqueous solution. J Phys Chem Ref Data. 1988;17:513–886. [Google Scholar]36. Christl SU, Murgatroyd PR, Gibson GR, Cummings JH. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992;102:1269–1277. [PubMed] [Google Scholar]37. Strocchi A, Levitt MD. Maintaining intestinal H2 balance: credit the colonic bacteria. Gastroenterology. 1992;102:1424–1426. [PubMed] [Google Scholar]38. Perman JA, Modler S, Barr RG, Rosenthal P. Fasting breath hydrogen concentration: normal values and clinical application. Gastroenterology. 1984;87(6):1358–1363. [PubMed] [Google Scholar]39. Sone Y, Tanida S, Matsubara K, et al. Everyday breath hydrogen excretion profile in Japanese young female students. J Physiol Anthropol Appl Human Sci. 2000;19:229–237. [PubMed] [Google Scholar]40. Nakao A, Kaczorowski DJ, Wang Y, et al. Amelioration of rat cardiac cold ischemia/reperfusion injury with inhaled hydrogen or carbon monoxide, or both. J Heart Lung Transplant. 2010;29:544–553. [PubMed] [Google Scholar]41. Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. [PMC free article] [PubMed] [Google Scholar]42. Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. [PMC free article] [PubMed] [Google Scholar]43. Gomez-Cabrera MC, Salvador-Pascual A, Cabo H, Ferrando B, Vina J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic Biol Med. 2015;86:37–46. [PubMed] [Google Scholar]44. Aoki K, Nakao A, Adachi T, Matsui Y, Miyakawa S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. 2012;2:12. [PMC free article] [PubMed] [Google Scholar]45. Han L, Tian R, Yan H, et al. Hydrogen-rich water protects against ischemic brain injury in rats by regulating calcium buffering proteins. Brain Res. 2015;1615:129–138. [PubMed] [Google Scholar]46. Matchett GA, Fathali N, Hasegawa Y, et al. Hydrogen gas is ineffective in moderate and severe neonatal hypoxia-ischemia rat models. Brain Res. 2009;1259:90–97. [PubMed] [Google Scholar]47. Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 2014;165:759–773. [PMC free article] [PubMed] [Google Scholar]48. Murakami Y, Ito M, Ohsawa I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One. 2017;12:e0176992. [PMC free article] [PubMed] [Google Scholar]49. Spulber S, Edoff K, Hong L, Morisawa S, Shirahata S, Ceccatelli S. Molecular hydrogen reduces LPS-induced neuroinflammation and promotes recovery from sickness behaviour in mice. PLoS One. 2012;7(7):e42078. [PMC free article] [PubMed] [Google Scholar]50. Kawamura T, Wakabayashi N, Shigemura N, et al. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am J Physiol Lung Cell Mol Physiol. 2013;304:646–656. [PMC free article] [PubMed] [Google Scholar]51. Zhai X, Chen X, Shi J, et al. Lactulose ameliorates cerebral ischemia-reperfusion injury in rats by inducing hydrogen by activating Nrf2 expression. Free Radic Biol Med. 2013;65:731–741. [PubMed] [Google Scholar]52. Li DZ, Zhang QX, Dong XX, Li HD, Ma X. Treatment with hydrogen molecules prevents RANKL-induced osteoclast differentiation associated with inhibition of ROS formation and inactivation of MAPK, AKT and NF-kappa B pathways in murine RAW264.7 cells. J Bone Miner Metab. 2014;32:494–504. [PubMed] [Google Scholar]53. Xie Q, Li XX, Zhang P, et al. Hydrogen gas protects against serum and glucose deprivationinduced myocardial injury in H9c2 cells through activation of the NFE2related factor 2/heme oxygenase 1 signaling pathway. Mol Med Rep. 2014;10:1143–1149. [PubMed] [Google Scholar]54. Song G, Zong C, Zhang Z, et al. Molecular hydrogen stabilizes atherosclerotic plaque in low-density lipoprotein receptor-knockout mice. Free Radic Biol Med. 2015;87:58–68. [PubMed] [Google Scholar]55. Li Y, Li Q, Chen H, et al. Hydrogen gas alleviates the intestinal injury caused by severe sepsis in mice by increasing the expression of heme oxygenase-1. Shock. 2015;44:90–98. [PubMed] [Google Scholar]56. Li Y, Xie K, Chen H, Wang G, Yu Y. Hydrogen gas inhibits high-mobility group box 1 release in septic mice by upregulation of heme oxygenase 1. J Surg Res. 2015;196:136–148. [PubMed] [Google Scholar]57. Huang CS, Kawamura T, Peng X, et al. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem Biophys Res Commun. 2011;408:253–258. [PubMed] [Google Scholar]58. Zhuang Z, Sun XJ, Zhang X, et al. Nuclear factor-kappaB/Bcl-XL pathway is involved in the protective effect of hydrogen-rich saline on the brain following experimental subarachnoid hemorrhage in rabbits. J Neurosci Res. 2013;91:1599–1608. [PubMed] [Google Scholar]59. Nishiwaki H, Ito M, Negishi S, Sobue S, Ichihara M, Ohno K. Molecular hydrogen upregulates heat shock response and collagen biosynthesis, and downregulates cell cycles: meta-analyses of gene expression profiles. Free Radic Res. 2018;52:434–445. [PubMed] [Google Scholar]



