Hydrogen gas inhalation in patients with CoronavirusScientific Research

Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial
Introduction
Coronavirus disease 2019 (COVID-19) has resulted in more than 8.7 million laboratory-confirmed cases and 460,000 deaths worldwide (1). Few, if any, therapies provide rapid relief of respiratory symptoms and prevention of disease progression. An important mechanism responsible for dyspnea and disease progression in patients with COVID-19 may be increased work of breathing due to increased airway resistance (2). Hydrogen/oxygen (H2-O2) gas inhalation may play a role in the treatment of COVID-19 due to its lower resistance compared to room air when passing through the respiratory tract.
MethodsOther Section
We recently conducted an open-label, multicenter clinical study of laboratory-confirmed COVID-19 patients from seven hospitals in China between January 21 and March 23, 2020. Patients ranged in age from 18 to 85 years and had dyspnea at admission and enrollment [see online supplement (http://dx.doi.org/10.21037/jtd-2020-057) for patient source, enrollment /Exclusion Criteria and Outcome Measures].
Due to the urgency of the treatment outbreak, randomization was not performed. Patients were assigned to treatment and control groups at the discretion of the treating physician. According to standard of care (3), patients in the treatment group were treated with a hydrogen/oxygen generator (model AMS-H-03, Shanghai Asclepius Meditec Co., Ltd., China) every day until discharge [see Supplement Online (http://dx.doi) .org/10.21037/jtd-2020-057) Figure E1]. Patients in the control group received only standard care (daily oxygen therapy) until discharge. The clinical assessment consisted of a five-category ordinal scale.
The entire analysis set was analyzed using R software version 3.5.1. This number (percentage) is used to summarize categorical variables and compare with chi-square or Fisher’s exact test. Relative risks (RR) were calculated with 95% confidence intervals (95% CI) to reflect the probability of the event in the treatment group. Continuous variables were expressed as mean ± standard deviation and compared using independent t-test or Wilcoxon rank-sum test. All tests were two-tailed and P<0.05 was considered statistically significant.
Results
Of the 633 patients screened, 215 and 328 patients, respectively, were excluded from the treatment and control groups due to deficiency [see Supplement Online (http://dx.doi.org/10.21037/jtd-2020-057 ) in Figure E2]. Dyspnea was present at enrollment. Finally, there were 44 cases in the treatment group and 46 cases in the control group. The median duration of H2-O2 and oxygen inhalation were 7.7 (interquartile range, 6.0-18.3) hours and 24 (interquartile range, 22.6-24.0) hours per day, respectively. Demographics and disease severity were comparable at baseline [see Tables E1, E2 in the online supplement (http://dx.doi.org/10.21037/jtd-2020-057)].
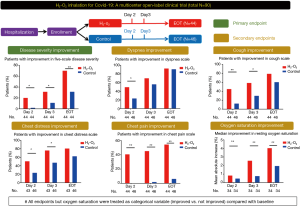
H2-O2 inhalation resulted in improved disease severity on day 2 in more patients (20.5% vs 2.3%, P=0.019; RR: 9.0, 95% CI: 1.2-68.1) and 3 (31.8% vs 11.5%, P=0.019; RR: 9.0, 95% CI: 1.2-68.1) = 0.038; RR: 2.8, 95% CI: 1.1-7.1) and end of treatment (70.5% vs. 31.8%, P<0.001, RR: 2.2, 95% CI: 1.4-3.6) (Figure 1). On day 2, the H2-O2 treatment group had greater improvement in the dyspnea scale (50.0% vs 23.9%, P=0.019; RR: 2.1, 95% CI: 1.2-3.8). Inhalation of H2-O2 improved chest discomfort and chest pain (both P<0.05). The treatment group had greater improvements in the cough scale on days 2 and 3 (both P<0.05). In addition, the improvement in resting oxygen saturation was greater after inhalation of H2-O2 (both P<0.05, Table 1).
Similar results were found when analyzing the outcome measures as continuous variables [all P<0.05, Supplementary Table E3 online (http://dx.doi.org/10.21037/jtd-2020-057)]. The improvement in the dyspnea scale at the end of treatment was more significant in the H2-O2 treatment group, regardless of baseline disease severity [Supplementary Table E4 online (http://dx.doi.org/10.21037/jtd-2020- 057)]. Patients who inhaled H2-O2 for less than the median duration (64 hours) still showed significant improvement [online Supplementary Table E5 (http://dx.doi.org/10.21037/jtd-2020-057)].
The most common side effects were increased cough (6.8% in the treatment group; 8.7% in the control group) and chest discomfort (2.3% in the treatment group; 21.7% in the control group). Abnormal laboratory findings were rare (2.3% in the treatment group; 13.0% in the control group). No serious adverse events were reported [Online Supplementary Form E6 (http://dx.doi.org/10.21037/jtd-2020-057)].
Discussion
This is the first multicenter randomized clinical trial to validate the efficacy and safety of H2-O2 inhalation in patients with COVID-19. The clinical benefit may be due to the ability of H2-O2 to reduce inspiratory effort due to significantly lower resistance through the airway compared to room air (previously validated by pulse oscillometric methods) (4). Patients with COVID-19 often experience dyspnea, cough, chest pain and discomfort, and oxygen desaturation (5) that other existing therapies, including oxygen therapy, do not rapidly improve. The effect of H2-O2 treatment became significant as early as day 2 and 3, and most of the improvement in respiratory symptoms persisted until the end of treatment, which also could not be easily explained by various supportive therapies including oxygen therapy .
Heliox inhalation has been reported to improve dyspnea and reduce airway resistance in adults and children (6,7). However, Heliox is not recommended for routine clinical use due to its low cost-effectiveness. H2-O2 can be produced by direct electrolysis of water using commercially available instruments, which has achieved clinical applications in homes and hospitals, especially in medical facilities with severe oxygen supply. Because of its safety profile, H2-O2 inhalation is particularly useful for relieving dyspnea and other respiratory symptoms in COVID-19 patients, regardless of the severity of the disease.
Our study was limited by the urgency of the open-label design and the variable duration of H2-O2 inhalation. Due to the urgency, we did not randomly assign patients with COVID-19, nor did we match patients with propensity scores, which may have biased selection. The H2-O2 inhalation protocol is created empirically and can guarantee optimization. Nonetheless, H2-O2 inhalation is useful for patients with dyspnea or in facilities without adequate oxygen supply.
Footnote
Provenance and Peer Review: In light of the revisions and high level of concern and urgency of research related to COVID-19, this short notice has been managed through expedited communication and has undergone internal review.
Conflicts of Interest: All authors have completed the Uniform ICMJE Disclosure Form (available at http://dx.doi.org/10.21037/jtd-2020-057). Professor Zhong Nanshan is the unpaid editor-in-chief of the Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethics Statement: The authors are responsible for all aspects of the work to ensure that issues related to the accuracy or completeness of any part of the work are properly investigated and resolved.
Open Access Statement: This is an open access article distributed under the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License (CC BY-NC-ND 4.0), which allows non-commercial reproduction of the article with strict restrictions and distribution, provided that no modification or editing has been made and the original work is properly cited (including links to official publications through the relevant DOI and license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- World Health Organization Coronavirus disease 2019 (COVID-19) Situation Reports. (accessed on June 22nd, 2020). Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref]
- National Health Commission. The Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5). (accessed on June 22nd, 2020). Available online: http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml
- Zhou ZQ, Zhong CH, Su ZQ, et al. Breathing Hydrogen-Oxygen Mixture Decreases Inspiratory Effort in Patients with Tracheal Stenosis. Respiration 2019;97:42-51. [Crossref]
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020. [Epub ahead of print].
- Morgan SE, Vukin K, Mosakowski S, et al. Use of heliox delivered via high-flow nasal cannula to treat an infant with coronavirus-related respiratory infection and severe acute air-flow obstruction. Respir Care 2014;59:e166-70. [Crossref]
- Kneyber MC, van Heerde M, Markhorst DG, et al. Mechanical ventilation with heliox decreases respiratory system resistance and facilitates CO2 removal in obstructive airway disease. Intensive Care Med 2006;32:1676-7. [Crossref]
DOI: 10.21037
Published on: 20201206
Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial
Introduction
Coronavirus disease 2019 (COVID-19) has resulted in more than 8.7 million laboratory-confirmed cases and 460,000 deaths worldwide (1). Few, if any, therapies provide rapid relief of respiratory symptoms and prevention of disease progression. An important mechanism responsible for dyspnea and disease progression in patients with COVID-19 may be increased work of breathing due to increased airway resistance (2). Hydrogen/oxygen (H2-O2) gas inhalation may play a role in the treatment of COVID-19 due to its lower resistance compared to room air when passing through the respiratory tract.
MethodsOther Section
We recently conducted an open-label, multicenter clinical study of laboratory-confirmed COVID-19 patients from seven hospitals in China between January 21 and March 23, 2020. Patients ranged in age from 18 to 85 years and had dyspnea at admission and enrollment [see online supplement (http://dx.doi.org/10.21037/jtd-2020-057) for patient source, enrollment /Exclusion Criteria and Outcome Measures].
Due to the urgency of the treatment outbreak, randomization was not performed. Patients were assigned to treatment and control groups at the discretion of the treating physician. According to standard of care (3), patients in the treatment group were treated with a hydrogen/oxygen generator (model AMS-H-03, Shanghai Asclepius Meditec Co., Ltd., China) every day until discharge [see Supplement Online (http://dx.doi) .org/10.21037/jtd-2020-057) Figure E1]. Patients in the control group received only standard care (daily oxygen therapy) until discharge. The clinical assessment consisted of a five-category ordinal scale.
The entire analysis set was analyzed using R software version 3.5.1. This number (percentage) is used to summarize categorical variables and compare with chi-square or Fisher’s exact test. Relative risks (RR) were calculated with 95% confidence intervals (95% CI) to reflect the probability of the event in the treatment group. Continuous variables were expressed as mean ± standard deviation and compared using independent t-test or Wilcoxon rank-sum test. All tests were two-tailed and P<0.05 was considered statistically significant.
Results
Of the 633 patients screened, 215 and 328 patients, respectively, were excluded from the treatment and control groups due to deficiency [see Supplement Online (http://dx.doi.org/10.21037/jtd-2020-057 ) in Figure E2]. Dyspnea was present at enrollment. Finally, there were 44 cases in the treatment group and 46 cases in the control group. The median duration of H2-O2 and oxygen inhalation were 7.7 (interquartile range, 6.0-18.3) hours and 24 (interquartile range, 22.6-24.0) hours per day, respectively. Demographics and disease severity were comparable at baseline [see Tables E1, E2 in the online supplement (http://dx.doi.org/10.21037/jtd-2020-057)].
H2-O2 inhalation resulted in improved disease severity on day 2 in more patients (20.5% vs 2.3%, P=0.019; RR: 9.0, 95% CI: 1.2-68.1) and 3 (31.8% vs 11.5%, P=0.019; RR: 9.0, 95% CI: 1.2-68.1) = 0.038; RR: 2.8, 95% CI: 1.1-7.1) and end of treatment (70.5% vs. 31.8%, P<0.001, RR: 2.2, 95% CI: 1.4-3.6) (Figure 1). On day 2, the H2-O2 treatment group had greater improvement in the dyspnea scale (50.0% vs 23.9%, P=0.019; RR: 2.1, 95% CI: 1.2-3.8). Inhalation of H2-O2 improved chest discomfort and chest pain (both P<0.05). The treatment group had greater improvements in the cough scale on days 2 and 3 (both P<0.05). In addition, the improvement in resting oxygen saturation was greater after inhalation of H2-O2 (both P<0.05, Table 1).
Figure 1 Study design and the main treatment effects of H2-O2 inhalation in patients with coronavirus disease 2019 who had dyspnea at enrollment. All endpoints but resting oxygen saturation were treated as categorical variables (improved vs. not improved) compared with the baseline levels. *, P<0.05; **, P<0.01. EOT, end-of-treatment, which was the day before hospital discharge.Table 1 Treatment effects in terms of the primary and secondary endpoints at different time pointsSimilar results were found when analyzing the outcome measures as continuous variables [all P<0.05, Supplementary Table E3 online (http://dx.doi.org/10.21037/jtd-2020-057)]. The improvement in the dyspnea scale at the end of treatment was more significant in the H2-O2 treatment group, regardless of baseline disease severity [Supplementary Table E4 online (http://dx.doi.org/10.21037/jtd-2020- 057)]. Patients who inhaled H2-O2 for less than the median duration (64 hours) still showed significant improvement [online Supplementary Table E5 (http://dx.doi.org/10.21037/jtd-2020-057)].
The most common side effects were increased cough (6.8% in the treatment group; 8.7% in the control group) and chest discomfort (2.3% in the treatment group; 21.7% in the control group). Abnormal laboratory findings were rare (2.3% in the treatment group; 13.0% in the control group). No serious adverse events were reported [Online Supplementary Form E6 (http://dx.doi.org/10.21037/jtd-2020-057)].
Discussion
This is the first multicenter randomized clinical trial to validate the efficacy and safety of H2-O2 inhalation in patients with COVID-19. The clinical benefit may be due to the ability of H2-O2 to reduce inspiratory effort due to significantly lower resistance through the airway compared to room air (previously validated by pulse oscillometric methods) (4). Patients with COVID-19 often experience dyspnea, cough, chest pain and discomfort, and oxygen desaturation (5) that other existing therapies, including oxygen therapy, do not rapidly improve. The effect of H2-O2 treatment became significant as early as day 2 and 3, and most of the improvement in respiratory symptoms persisted until the end of treatment, which also could not be easily explained by various supportive therapies including oxygen therapy .
Heliox inhalation has been reported to improve dyspnea and reduce airway resistance in adults and children (6,7). However, Heliox is not recommended for routine clinical use due to its low cost-effectiveness. H2-O2 can be produced by direct electrolysis of water using commercially available instruments, which has achieved clinical applications in homes and hospitals, especially in medical facilities with severe oxygen supply. Because of its safety profile, H2-O2 inhalation is particularly useful for relieving dyspnea and other respiratory symptoms in COVID-19 patients, regardless of the severity of the disease.
Our study was limited by the urgency of the open-label design and the variable duration of H2-O2 inhalation. Due to the urgency, we did not randomly assign patients with COVID-19, nor did we match patients with propensity scores, which may have biased selection. The H2-O2 inhalation protocol is created empirically and can guarantee optimization. Nonetheless, H2-O2 inhalation is useful for patients with dyspnea or in facilities without adequate oxygen supply.
Footnote
Provenance and Peer Review: In light of the revisions and high level of concern and urgency of research related to COVID-19, this short notice has been managed through expedited communication and has undergone internal review.
Conflicts of Interest: All authors have completed the Uniform ICMJE Disclosure Form (available at http://dx.doi.org/10.21037/jtd-2020-057). Professor Zhong Nanshan is the unpaid editor-in-chief of the Journal of Thoracic Disease. The other authors have no conflicts of interest to declare.
Ethics Statement: The authors are responsible for all aspects of the work to ensure that issues related to the accuracy or completeness of any part of the work are properly investigated and resolved.
Open Access Statement: This is an open access article distributed under the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License (CC BY-NC-ND 4.0), which allows non-commercial reproduction of the article with strict restrictions and distribution, provided that no modification or editing has been made and the original work is properly cited (including links to official publications through the relevant DOI and license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- World Health Organization Coronavirus disease 2019 (COVID-19) Situation Reports. (accessed on June 22nd, 2020). Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref]
- National Health Commission. The Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5). (accessed on June 22nd, 2020). Available online: http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml
- Zhou ZQ, Zhong CH, Su ZQ, et al. Breathing Hydrogen-Oxygen Mixture Decreases Inspiratory Effort in Patients with Tracheal Stenosis. Respiration 2019;97:42-51. [Crossref]
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020. [Epub ahead of print].
- Morgan SE, Vukin K, Mosakowski S, et al. Use of heliox delivered via high-flow nasal cannula to treat an infant with coronavirus-related respiratory infection and severe acute air-flow obstruction. Respir Care 2014;59:e166-70. [Crossref]
- Kneyber MC, van Heerde M, Markhorst DG, et al. Mechanical ventilation with heliox decreases respiratory system resistance and facilitates CO2 removal in obstructive airway disease. Intensive Care Med 2006;32:1676-7. [Crossref]
Cite this article as: Guan WJ, Wei CH, Chen AL, Sun XC, Guo GY, Zou X, Shi JD, Lai PZ, Zheng ZG, Zhong NS. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J Thorac Dis 2020;12(6):3448-3452. doi: 10.21037/jtd-2020-057