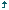Hydrogen-controlled cancerScientific Research

“Real world survey” of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients
Ji-Bing Chen1, Xiao-Feng Kong2, You-Yong Lv3, Shu-Cun Qin4, Xue-Jun Sun5, Feng Mu1, Tian-Yu Lu6, Ke-Cheng Xu6
1 Department of Cancer Rehabilitation, Fuda Cancer Hospital of Jinan University, Guangzhou, Guangdong Province, China
2 Research Center of Hydrogen Medicine, Xukecheng Health Care Studio of Guangdong Province, Guangzhou, Guangdong Province, China
3 Molecular Biology Laboratory of Cancer Hospital, Peking University, Beijing, China
4 Institute of Hydrogen Medicine, Shandong Medical University, Jinan, Shandong Province, China
5 Institute of Diving Medicine, Navy Medical University, Shanghai, China
6 Department of Cancer Rehabilitation, Fuda Cancer Hospital of Jinan University; Research Center of Hydrogen Medicine, Xukecheng Health Care Studio of Guangdong Province, Guangzhou, Guangdong Province, China
Abstract
Advanced cancer treatments are challenging and require new ideas and strategies. Hydrogen exerts antioxidant and anti-inflammatory effects and can be used to fight cancer, whose pathogenesis and progression are closely related to peroxidation and inflammation. We conducted a prospective follow-up study of 82 patients with stage III and IV cancer treated with hydrogen inhalation using real-world evidence. After 3-46 months of follow-up, 12 stage IV patients died. After 4 weeks of hydrogen inhalation, patients reported significant improvements in fatigue, insomnia, anorexia, and pain. In addition, 41.5% of patients showed improvement in physical condition, with the best results in lung cancer patients and the worst results in pancreatic cancer and gynecological cancer patients. Of the 58 cases with elevated one or more abnormal tumor markers, 36.2% had marker decreases 13-45 days (median 23 days) after hydrogen inhalation. The greatest marker reductions were lowest in lung cancer and pancreatic and liver malignancies. Among the 80 patients with radiographically visible tumors, the overall disease control rate was 57.5%, with complete and partial responses occurring 21-80 days (median 55 days) after hydrogen inhalation. The disease control rate of patients with stage III was significantly higher than that of patients with stage IV (83.0% and 47.7%, respectively), and the disease control rate of patients with pancreatic cancer was the lowest. No hematologic toxicity was observed, although minor side effects with spontaneous resolution were observed in individual cases. In patients with advanced cancer, inhaled hydrogen can improve the patient’s quality of life and control cancer progression. Hydrogen inhalation is a simple and inexpensive treatment with few side effects and deserves further study as a strategy for clinical rehabilitation of advanced cancer patients. The research protocol was approved by the Ethics Committee of the Ethics Committee of Fuda Cancer Hospital of Jinan University on December 7, 2018 (approval number: Fuda20181207).
Results
Clinical data of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
A total of 82 patients were followed up (14 at home and 68 patients in our clinic). The clinicopathological data of patients who received hydrogen inhalation therapy are shown in [Table 1].
Table 1: Clinicopathological data of advanced cancer patients who received hydrogen inhalation therapy.
Follow-up time and survival of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
All patients were followed up for 3-46 months, with a median of 6 months. Forty-five patients (55%) were followed for 3-6 months, 33 (40%) were followed for 7-12 months, and only 1 was 13, 14, 26, and 46 months. Twelve patients died (all stage IV), 4 pancreatic cancers (all tumor progression), 2 liver cancers (1 each for liver failure and infection), and 1 lung cancer (programmed cell death protein-1 (PD-1). 1) Antibody-related pneumonia), 3 cases of gynecological malignancies (1 case each of intestinal obstruction, abdominal infection and tumor progression), 1 case of gastrointestinal malignancy (hemorrhage of upper gastrointestinal tract), and 1 additional case (infection).
Quality-of-life
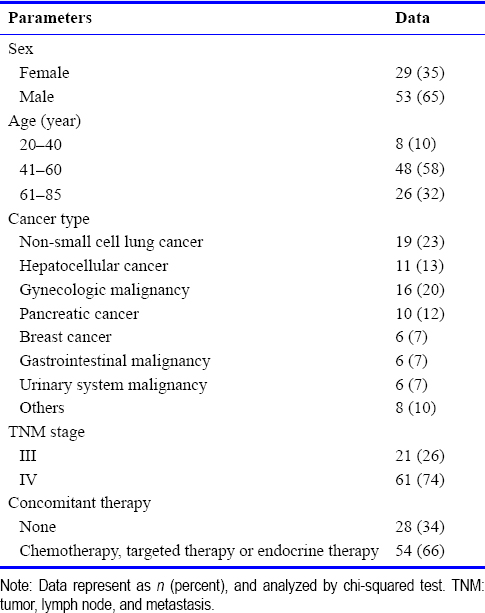
Thirty patients were prospectively evaluated using the QLQ-C30 score. After 2 weeks of hydrogen inhalation, patients reported decreased dyspnea, increased appetite, significant improvements in physical, role-related, and emotional functioning, and decreased fatigue, nausea, vomiting, and insomnia. After 4 weeks of treatment, there were significant improvements in cognitive function, pain, appetite, constipation, and diarrhea [Table 2].
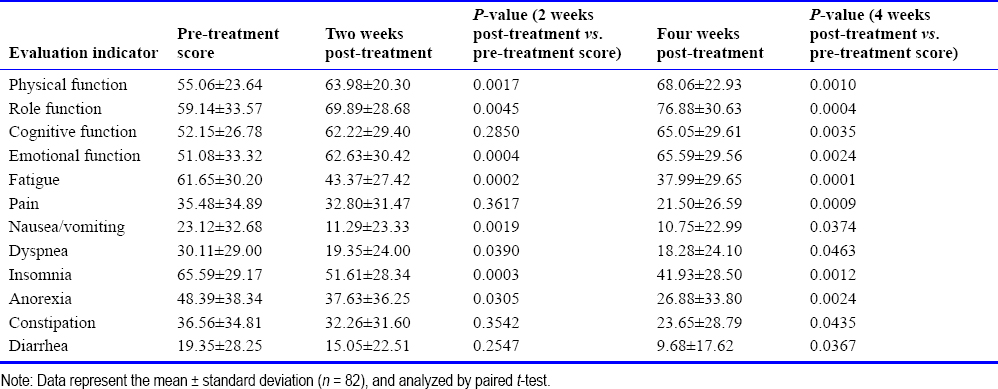
Table 2: Quality-of-life scores of advanced cancer patients who received hydrogen inhalation therapy
Physical fitness evaluation
The physical fitness of the patients was assessed using the Zubrod ECOG-WHO scoring system. After 3 months of hydrogen inhalation therapy, 42% of patients improved, 34% stabilized, and 24% worsened. The improvement rate of patients who received hydrogen inhalation therapy alone was 32%, and the improvement rate of patients who received hydrogen inhalation therapy combined with other treatments was 44%; there was no significant difference between the two groups (P=0.295). Compared with pretreatment, the improvement rate was 57% in stage III patients and 36% in stage IV patients, with no significant difference between groups (p=0.572). There are differences in the improvement of physical fitness of patients with different tumor types. Lung cancer patients showed the greatest improvement in physical fitness (68%), while pancreatic cancer patients (0%) and gynecologic oncology patients (12%) showed the smallest improvements. Improvement was significantly greater in patients with lung cancer than in patients with gynecologic and pancreatic cancer [Table 3].
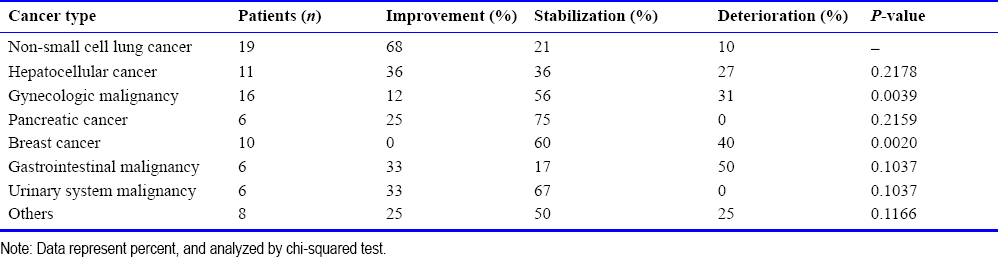
Table 3: Physical status of advanced cancer patients with different cancer types
Changes in tumor markers of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
A total of 58 patients had elevated tumor markers before hydrogen therapy. After three months of hydrogen treatment, tumor markers decreased in 36% of patients, unchanged in 16%, and increased in 48%. A typical case of tumor marker reduction is shown in [Figure 1]. The initial reduction in tumor markers occurred within a period of 13-45 days (median 23 days) after treatment. Compared with before treatment, the expression levels of tumor markers in patients receiving hydrogen inhalation therapy alone and patients receiving combined therapy were decreased by 22% and 42%, respectively, and the difference between the two groups was not statistically significant (P = 0, 3131) . In patients with normal tumor markers before treatment, no increase in tumor markers was observed after hydrogen inhalation therapy. Fifty-eight patients with elevated biomarkers were analyzed by tumor type. After 3 months of hydrogen inhalation therapy, patients with lung cancer showed the greatest decrease in tumor markers (75%), whereas no decrease was observed in patients with pancreatic and liver cancer [Table 4]. There were significant differences in the changes of tumor markers between lung cancer patients and gynecological, liver cancer, and pancreatic cancer patients after treatment.
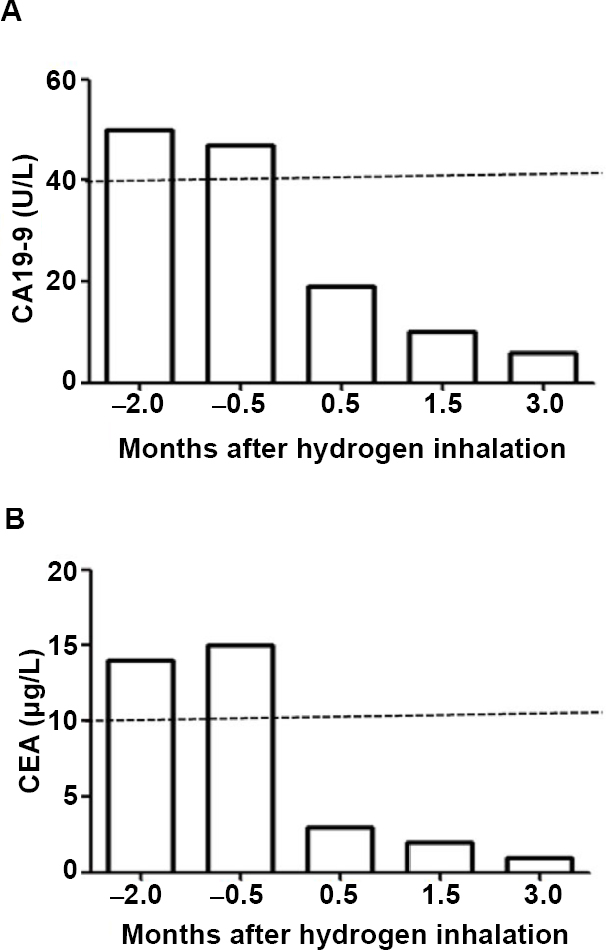
Figure 1: The tumor markers were decreased in the typical advanced cancer cases treated with hydrogen inhalation rehabilitation.
Note: (A, B) Miss Y, 28 years old. The uterus and attachments were removed because the left ovarian cancer in December 2017. After 3 months, the tumor marker examination was elevated twice, and there were no symptoms on computed tomography, ultrasound, and endoscopy. Hydrogen inhalation began on May 9, 2018, 3–4 hours daily. After 2 weeks, the tumor markers CA19-9 (A) and carcinoembryonic antigen (CEA) (B) were reviewed and completely reduced to normal (dotted lines in the figure). Until the beginning of 2019, there was no recurrence.
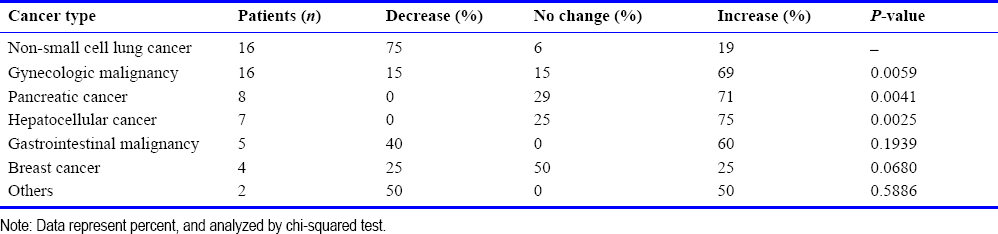
Table 4: Changes in tumor markers in advanced cancer patients with different tumor types
Tumor response of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
Of the 82 patients enrolled in the study, 80 had tumors prior to enrollment, and the other two had only elevated tumor markers. Of the 80 tumor patients, 1 (1%) CR, 15 (19%) PR, 30 (38%) and 34 (42%) progressed after 3 months of hydrogen inhalation therapy, with a DCR of 57.5% A typical case of tumor shrinkage is shown in Figure 2. CR and PR occurred within 21-80 days, with a median of 55 days.The DCR of patients who received hydrogen inhalation therapy alone was 54% and that of patients who received hydrogen inhalation therapy combined with other treatments, respectively, and there was no significant difference in DCR between the two groups (P=0.5917). From a tumor stage perspective, DCR was significantly higher in stage III patients (83%) than in stage IV patients (48%) [Table 5]. Lung cancer patients had a higher DCR (79%), while pancreatic cancer patients had the lowest DCR (20%) and were the only group with a significant difference in DCR compared to lung cancer patients (p=0.0161) . .
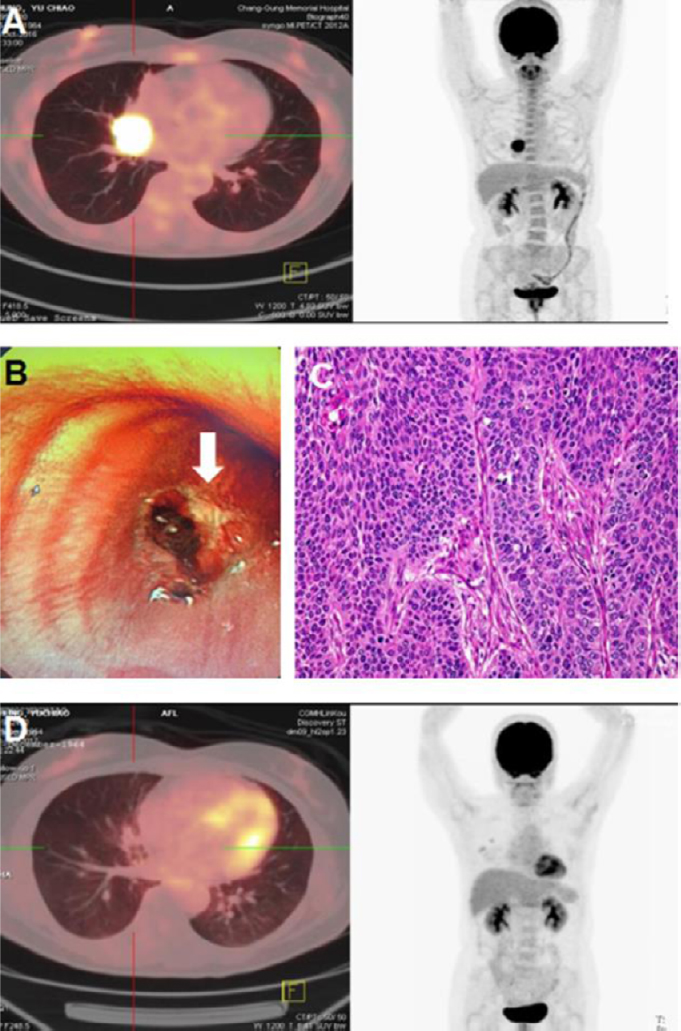
Figure 2: The tumor reduction in the typical advanced cancer cases treated with hydrogen inhalation rehabilitation.
Note: (A) Miss Z, 52-year-old, had irritable cough and was diagnosed with positron emission tomography (PET)-computed tomography (CT) scanning in October 27, 2016, showed right middle lung mass (4.1 cm × 3.9 cm) with hilar invasion. (B, C) Bronchial endoscopy showed that a hemorrhagic-erosive lesion was located at the right main bronchus (white arrow), and biopsy showed poorly-differentiated squamous cell carcinoma (C). (D) The patient refused chemotherapy and inhaled hydrogen from November 4, 2016, at least 4 hours a day. After 1 week, the cough was reduced and the breathing became smooth. After 2.5 months, on January 13, 2017, PET-CT was performed and the original lesions in the lungs were not seen. So far, the patient has disease-free survived up to now.

Table 5: Tumor response of stage III and IV advanced cancer patients with hydrogen inhalation rehabilitation
Side-effects of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
Among the 29 patients who received hydrogen inhalation alone, no hematologic toxicities were found (routine blood test data not shown), 1 patient experienced stomach pain and chills, and 1 patient experienced dizziness (spontaneous resolution after a few days). Of the 53 patients who received combined hydrogen inhalation therapy, 1 patient experienced headache (which resolved spontaneously within 3-5 days), 1 patient experienced occasional epistaxis (minor bleeding that gradually resolved), and 1 patient reported nasal dryness, inhalation Disappeared after a few hours of cessation.
References
| 1. | |
| 2. | |
| 3. | |
| 4. | |
| 5. | |
| 6. | |
| 7. | |
| 8. | |
| 9. | |
| 10. | |
| 11. | |
| 12. | |
| 13. | |
| 14. | |
| 15. | |
| 16. | |
| 17. | |
| 18. | |
| 19. | |
| 20. | |
| 21. | |
| 22. | |
| 23. | |
| 24. | |
| 25. | |
| 26. | |
| 27. | |
| 28. | |
| 29. | |
| 30. | |
| 31. | |
| 32. | |
| 33. | |
| 34. | |
| 35. | |
| 36. | |
| 37. | |
| 38. |
Published on: 24/05/2023
“Real world survey” of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients
Ji-Bing Chen1, Xiao-Feng Kong2, You-Yong Lv3, Shu-Cun Qin4, Xue-Jun Sun5, Feng Mu1, Tian-Yu Lu6, Ke-Cheng Xu6
1 Department of Cancer Rehabilitation, Fuda Cancer Hospital of Jinan University, Guangzhou, Guangdong Province, China
2 Research Center of Hydrogen Medicine, Xukecheng Health Care Studio of Guangdong Province, Guangzhou, Guangdong Province, China
3 Molecular Biology Laboratory of Cancer Hospital, Peking University, Beijing, China
4 Institute of Hydrogen Medicine, Shandong Medical University, Jinan, Shandong Province, China
5 Institute of Diving Medicine, Navy Medical University, Shanghai, China
6 Department of Cancer Rehabilitation, Fuda Cancer Hospital of Jinan University; Research Center of Hydrogen Medicine, Xukecheng Health Care Studio of Guangdong Province, Guangzhou, Guangdong Province, ChinaAbstract
Advanced cancer treatments are challenging and require new ideas and strategies. Hydrogen exerts antioxidant and anti-inflammatory effects and can be used to fight cancer, whose pathogenesis and progression are closely related to peroxidation and inflammation. We conducted a prospective follow-up study of 82 patients with stage III and IV cancer treated with hydrogen inhalation using real-world evidence. After 3-46 months of follow-up, 12 stage IV patients died. After 4 weeks of hydrogen inhalation, patients reported significant improvements in fatigue, insomnia, anorexia, and pain. In addition, 41.5% of patients showed improvement in physical condition, with the best results in lung cancer patients and the worst results in pancreatic cancer and gynecological cancer patients. Of the 58 cases with elevated one or more abnormal tumor markers, 36.2% had marker decreases 13-45 days (median 23 days) after hydrogen inhalation. The greatest marker reductions were lowest in lung cancer and pancreatic and liver malignancies. Among the 80 patients with radiographically visible tumors, the overall disease control rate was 57.5%, with complete and partial responses occurring 21-80 days (median 55 days) after hydrogen inhalation. The disease control rate of patients with stage III was significantly higher than that of patients with stage IV (83.0% and 47.7%, respectively), and the disease control rate of patients with pancreatic cancer was the lowest. No hematologic toxicity was observed, although minor side effects with spontaneous resolution were observed in individual cases. In patients with advanced cancer, inhaled hydrogen can improve the patient’s quality of life and control cancer progression. Hydrogen inhalation is a simple and inexpensive treatment with few side effects and deserves further study as a strategy for clinical rehabilitation of advanced cancer patients. The research protocol was approved by the Ethics Committee of the Ethics Committee of Fuda Cancer Hospital of Jinan University on December 7, 2018 (approval number: Fuda20181207).
Results
Clinical data of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
A total of 82 patients were followed up (14 at home and 68 patients in our clinic). The clinicopathological data of patients who received hydrogen inhalation therapy are shown in [Table 1].
Table 1: Clinicopathological data of advanced cancer patients who received hydrogen inhalation therapy.
Follow-up time and survival of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
All patients were followed up for 3-46 months, with a median of 6 months. Forty-five patients (55%) were followed for 3-6 months, 33 (40%) were followed for 7-12 months, and only 1 was 13, 14, 26, and 46 months. Twelve patients died (all stage IV), 4 pancreatic cancers (all tumor progression), 2 liver cancers (1 each for liver failure and infection), and 1 lung cancer (programmed cell death protein-1 (PD-1). 1) Antibody-related pneumonia), 3 cases of gynecological malignancies (1 case each of intestinal obstruction, abdominal infection and tumor progression), 1 case of gastrointestinal malignancy (hemorrhage of upper gastrointestinal tract), and 1 additional case (infection).
Quality-of-life
Thirty patients were prospectively evaluated using the QLQ-C30 score. After 2 weeks of hydrogen inhalation, patients reported decreased dyspnea, increased appetite, significant improvements in physical, role-related, and emotional functioning, and decreased fatigue, nausea, vomiting, and insomnia. After 4 weeks of treatment, there were significant improvements in cognitive function, pain, appetite, constipation, and diarrhea [Table 2].
Table 2: Quality-of-life scores of advanced cancer patients who received hydrogen inhalation therapy
Physical fitness evaluation
The physical fitness of the patients was assessed using the Zubrod ECOG-WHO scoring system. After 3 months of hydrogen inhalation therapy, 42% of patients improved, 34% stabilized, and 24% worsened. The improvement rate of patients who received hydrogen inhalation therapy alone was 32%, and the improvement rate of patients who received hydrogen inhalation therapy combined with other treatments was 44%; there was no significant difference between the two groups (P=0.295). Compared with pretreatment, the improvement rate was 57% in stage III patients and 36% in stage IV patients, with no significant difference between groups (p=0.572). There are differences in the improvement of physical fitness of patients with different tumor types. Lung cancer patients showed the greatest improvement in physical fitness (68%), while pancreatic cancer patients (0%) and gynecologic oncology patients (12%) showed the smallest improvements. Improvement was significantly greater in patients with lung cancer than in patients with gynecologic and pancreatic cancer [Table 3].
Table 3: Physical status of advanced cancer patients with different cancer types
Changes in tumor markers of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
A total of 58 patients had elevated tumor markers before hydrogen therapy. After three months of hydrogen treatment, tumor markers decreased in 36% of patients, unchanged in 16%, and increased in 48%. A typical case of tumor marker reduction is shown in [Figure 1]. The initial reduction in tumor markers occurred within a period of 13-45 days (median 23 days) after treatment. Compared with before treatment, the expression levels of tumor markers in patients receiving hydrogen inhalation therapy alone and patients receiving combined therapy were decreased by 22% and 42%, respectively, and the difference between the two groups was not statistically significant (P = 0, 3131) . In patients with normal tumor markers before treatment, no increase in tumor markers was observed after hydrogen inhalation therapy. Fifty-eight patients with elevated biomarkers were analyzed by tumor type. After 3 months of hydrogen inhalation therapy, patients with lung cancer showed the greatest decrease in tumor markers (75%), whereas no decrease was observed in patients with pancreatic and liver cancer [Table 4]. There were significant differences in the changes of tumor markers between lung cancer patients and gynecological, liver cancer, and pancreatic cancer patients after treatment.
Figure 1: The tumor markers were decreased in the typical advanced cancer cases treated with hydrogen inhalation rehabilitation.
Note: (A, B) Miss Y, 28 years old. The uterus and attachments were removed because the left ovarian cancer in December 2017. After 3 months, the tumor marker examination was elevated twice, and there were no symptoms on computed tomography, ultrasound, and endoscopy. Hydrogen inhalation began on May 9, 2018, 3–4 hours daily. After 2 weeks, the tumor markers CA19-9 (A) and carcinoembryonic antigen (CEA) (B) were reviewed and completely reduced to normal (dotted lines in the figure). Until the beginning of 2019, there was no recurrence.
Table 4: Changes in tumor markers in advanced cancer patients with different tumor types
Tumor response of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
Of the 82 patients enrolled in the study, 80 had tumors prior to enrollment, and the other two had only elevated tumor markers. Of the 80 tumor patients, 1 (1%) CR, 15 (19%) PR, 30 (38%) and 34 (42%) progressed after 3 months of hydrogen inhalation therapy, with a DCR of 57.5% A typical case of tumor shrinkage is shown in Figure 2. CR and PR occurred within 21-80 days, with a median of 55 days.The DCR of patients who received hydrogen inhalation therapy alone was 54% and that of patients who received hydrogen inhalation therapy combined with other treatments, respectively, and there was no significant difference in DCR between the two groups (P=0.5917). From a tumor stage perspective, DCR was significantly higher in stage III patients (83%) than in stage IV patients (48%) [Table 5]. Lung cancer patients had a higher DCR (79%), while pancreatic cancer patients had the lowest DCR (20%) and were the only group with a significant difference in DCR compared to lung cancer patients (p=0.0161) . .
Figure 2: The tumor reduction in the typical advanced cancer cases treated with hydrogen inhalation rehabilitation.
Note: (A) Miss Z, 52-year-old, had irritable cough and was diagnosed with positron emission tomography (PET)-computed tomography (CT) scanning in October 27, 2016, showed right middle lung mass (4.1 cm × 3.9 cm) with hilar invasion. (B, C) Bronchial endoscopy showed that a hemorrhagic-erosive lesion was located at the right main bronchus (white arrow), and biopsy showed poorly-differentiated squamous cell carcinoma (C). (D) The patient refused chemotherapy and inhaled hydrogen from November 4, 2016, at least 4 hours a day. After 1 week, the cough was reduced and the breathing became smooth. After 2.5 months, on January 13, 2017, PET-CT was performed and the original lesions in the lungs were not seen. So far, the patient has disease-free survived up to now.
Table 5: Tumor response of stage III and IV advanced cancer patients with hydrogen inhalation rehabilitation
Side-effects of 82 advanced cancer patients treated with hydrogen inhalation rehabilitation
Among the 29 patients who received hydrogen inhalation alone, no hematologic toxicities were found (routine blood test data not shown), 1 patient experienced stomach pain and chills, and 1 patient experienced dizziness (spontaneous resolution after a few days). Of the 53 patients who received combined hydrogen inhalation therapy, 1 patient experienced headache (which resolved spontaneously within 3-5 days), 1 patient experienced occasional epistaxis (minor bleeding that gradually resolved), and 1 patient reported nasal dryness, inhalation Disappeared after a few hours of cessation.
References
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38.