Hydrogen kills cancer cells in the lungScientific Research
Suppression of autophagy facilitates hydrogen gas‑mediated lung cancer cell apoptosis
Abstract
Our previous study found that hydrogen (H2) could effectively inhibit the progression of lung cancer; however, the underlying mechanism has not been elucidated. This study aimed to investigate the role of H2 in autophagy of lung cancer cells, and to reveal the effect of autophagy on H2-mediated apoptosis of lung cancer cells and its underlying mechanism. Expression levels of proteins associated with apoptosis and autophagy were detected using western blot analysis. Autophagy was inhibited by 3-methyladenine treatment or downregulation of Beclin1, while rapamycin was used to induce autophagy. Cell growth and apoptosis were detected using Cell Counting Kit-8 and flow cytometry, respectively. The results showed that apoptosis and autophagy were significantly enhanced in H2-treated A549 and H1975 lung cancer cell lines. However, enhanced autophagy attenuated the apoptosis-promoting effect of H2 and vice versa. Furthermore, it was found that H2 treatment induced a significant decrease in the protein expression levels of phosphorylated STAT3 and Bcl2, and overexpression of STAT3 abolished the role of H2 in promoting apoptosis and autophagy. In conclusion, this study showed that H2 could promote apoptosis and autophagy in lung cancer cells by inhibiting the activation of STAT3/Bcl2 signaling pathway, and inhibiting autophagy could enhance the role of H2 in promoting lung cancer cell apoptosis.
Results
H2 treatment induces a marked increase in apoptosis and autophagy
This study first examined the effects of H2 treatment on apoptosis and autophagy in lung cancer cells. Following H2 treatment in A549 and H1975 cells, cell viability was decreased in a time-dependent manner (Fig. 1A), while apoptotic rates were increased (Fig. 1B and C) compared to controls. The expression levels of pro-apoptotic proteins, including cleaved caspase 3 and cleaved PARP, were significantly increased in both cell lines after H2 treatment in a dose-dependent manner compared to the control group (Fig. 1D and E) (Fig. 1D and E). In addition, Beclin1 expression and LC3BII/I ratio were also significantly increased after H2 treatment, while p62 expression was significantly decreased (Figure 1D and E), also in a dose-dependent manner. These results suggest that H2 can induce apoptosis and autophagy in lung cancer cell lines.
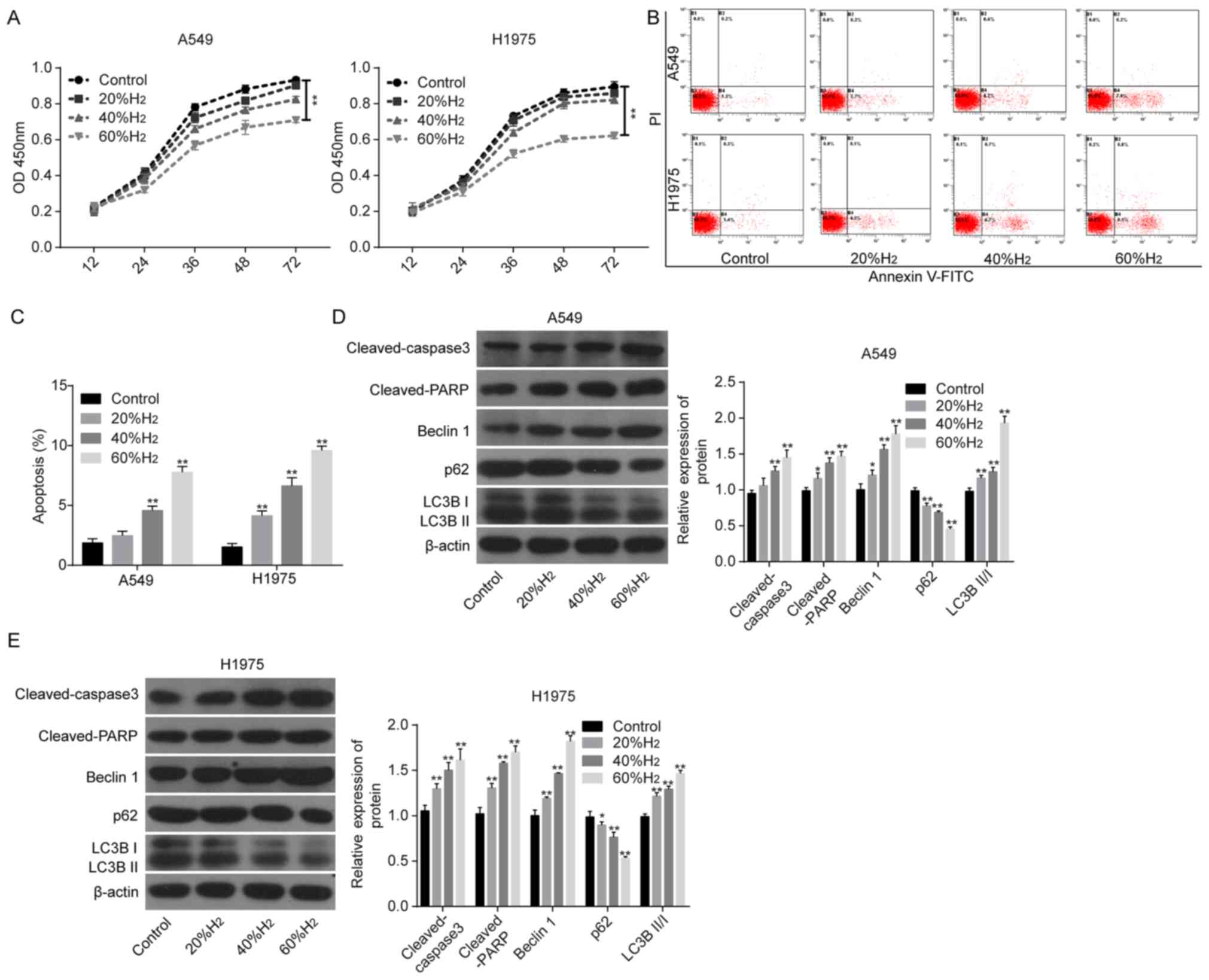
Figure 1. – H2 treatment enhances lung cancer cell apoptosis and autophagy. After A549 and H1975 cells were treated with 20, 40 or 60% H2, the following assays were performed. (A) Cell Counting Kit-8 assay was used to investigate cell growth. (B and C) Flow cytometry assay was used to detect cell apoptosis. (D and E) The protein levels of cleaved-caspase 3, cleaved-PARP, p62, Beclin1, LC3BII and LC3BI were detected using western blot analysis. *P<0.05 and **P<0.01 vs. control. PARP, poly ADP-ribose polymerase; LC3, light chain 3; H2, hydrogen gas.
Autophagy weakens the role of H2 in promoting cell apoptosis in lung cancer cells
Subsequently, the role of autophagy in H2-induced apoptosis of lung cancer cells was examined. When A549 and H1975 cells were treated with the autophagy inducer RAPA, the protein expression levels of Beclin1 and LC3BII/I were significantly increased, while that of p62 was decreased (Figure 2A). Since 60% H2 caused significant changes in cell viability and apoptosis, 60% H2 was chosen for subsequent experiments. After RAPA treatment, the apoptosis rate (Fig. 2B) and the protein expression levels of cleaved caspase 3 and cleaved PARP (Fig. 2C and D) were significantly decreased compared with the H2 group. In contrast, the protein expression levels of Beclin1 and LC3BII/I were significantly decreased and p62 expression was increased after cells were treated with the autophagy inhibitor 3-MA (Figure 2E). Furthermore, 3-MA treatment enhanced H2-mediated increase in apoptosis rates and the protein expression levels of cleaved caspase 3 and cleaved PARP compared to the H2 group (Fig. 2G and H). These results suggest that autophagy attenuates the effect of H2 in inducing apoptosis in lung cancer cells.
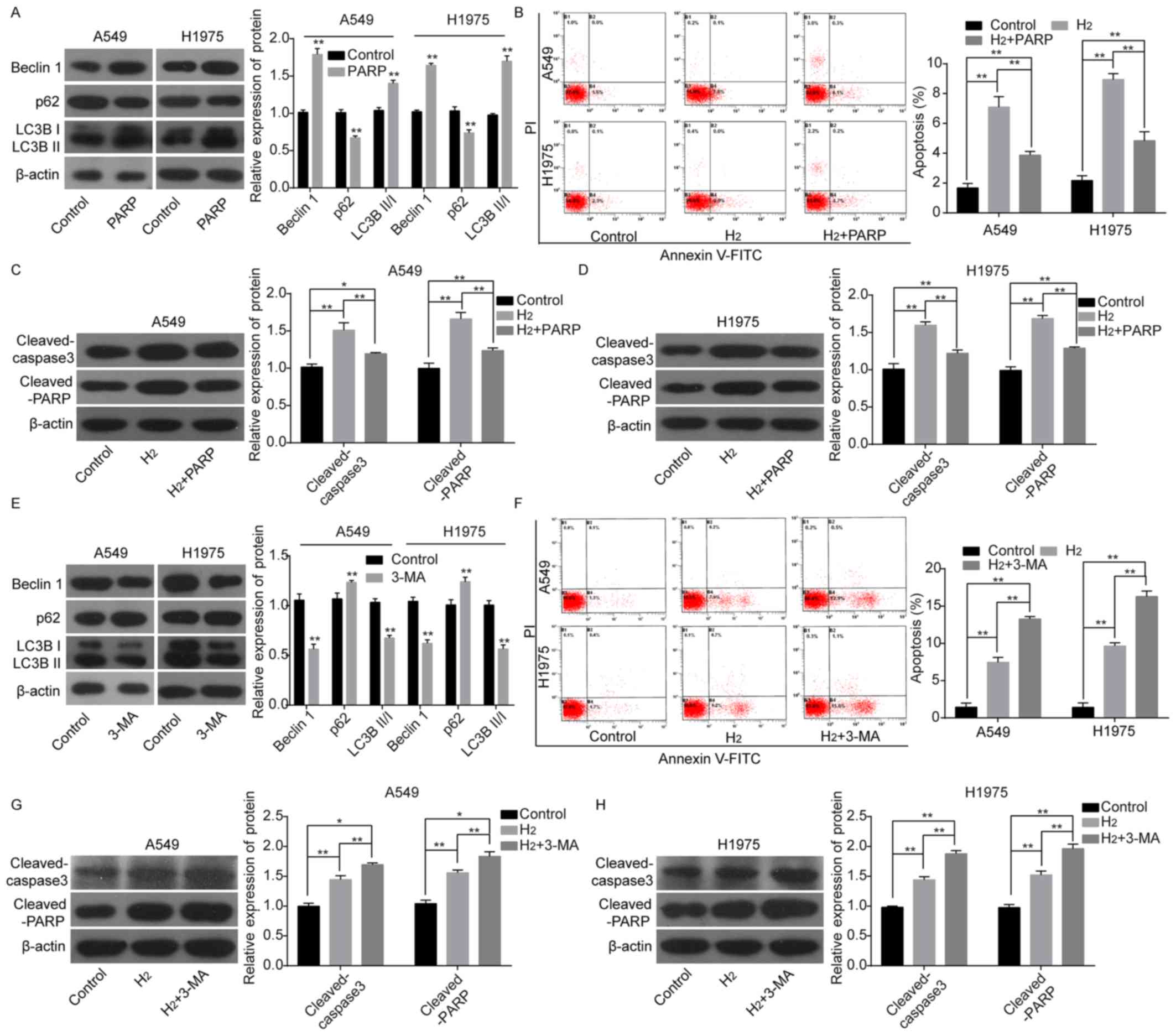
Figure 2. – Evaluation of autophagy effects on H2-induced lung cancer cell apoptosis. (A) The protein expression levels of p62, Beclin1, LC3BII and LC3BI, following treatment of A549 and H1975 cells with RAPA were detected using western blot analysis. (B) Cell apoptosis was investigated using a flow cytometry assay. Western blot analysis of the expression of cleaved-caspase 3 and cleaved-PARP in (C) A549 and (D) H1975 cells treated with H2 or H2+RAPA. (E) The protein expression levels of p62, Beclin1, LC3BII and LC3BI were detected using western blot analysis following treatment of A549 and H1975 cells with 3-MA. (F) Cell apoptosis was investigated using flow cytometry. Western blot analysis of the protein expression levels of cleaved-caspase 3 and cleaved-PARP following treatment of (G) A549 and (H) H1975 cells with H2 or H2+3-MA. *P<0.05. **P<0.01. LC3, light chain 3; RAPA, rapamycin; PARP, poly ADP-ribose polymerase; PI, propidium iodide; 3-MA, 3-methyladenine; H2, hydrogen gas.
Knockdown of Beclin1 enhances the roles of H2 in promoting cell apoptosis in lung cancer cell lines
To further elucidate the role of autophagy in H2-mediated apoptosis in lung cancer cells, knockdown experiments were performed. Beclin1 expression was significantly reduced in A549 and H1975 cells transfected with siRNA targeting the human Beclin1 gene. Since si-Beclin1-3 significantly reduced the protein expression level of Beclin1 in the 3 siRNAs (Figure 3A), it was selected for subsequent experiments. After cells were transfected with si-Beclin1-3, the protein expression level of p62 was increased and the expression ratio of LC3BII:LC3BI was decreased (Figure 3B and C). Notably, H2 treatment-induced apoptosis was significantly increased when Beclin1 was silenced in A549 and H1975 cells compared with the H2 group (Fig. 3D).
Furthermore, knockdown of Beclin1 increased the protein expression levels of cleaved caspase 3 and cleaved PARP in A549 and H1975 cells after H2 treatment compared to cells treated with H2 alone (Figure 3E and F) . These results further confirmed that inhibition of autophagy could enhance the effect of H2 on apoptosis induction in lung cancer cell lines.
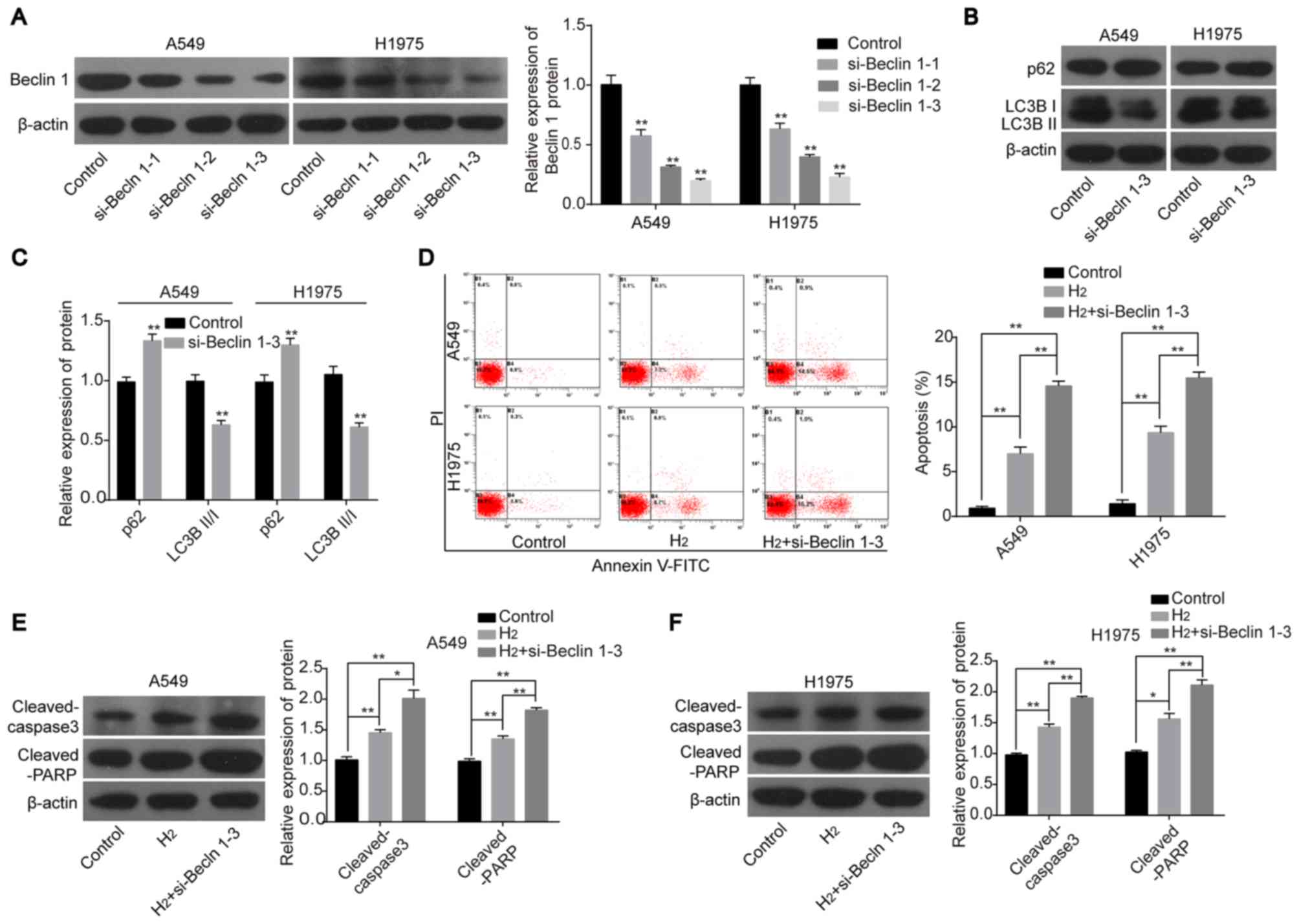
Figure 3. – Knockdown of Beclin1 enhances the role of H2 in promoting lung cancer cell apoptosis. (A) The knockdown efficiency of si-Beclin1 in A549 and H1975 cells was detected using western blot analysis. (B) Western blot analysis was used to detect the protein expression levels of p62, LC3BII and LC3BI in A549 and H1975 cells and the results were subsequently (C) quantified. (D) Flow cytometry assay was used to investigate cell apoptosis. The protein expression levels of cleaved-caspase 3 and cleaved-PARP were determined using western blot analysis in (E) A549 and (F) H1975 cells. *P<0.05 and **P<0.01. si, short interfering; LC3, light chain 3; PARP, poly ADP-ribose polymerase; PI, propidium iodide; H2, hydrogen gas.
H2 treatment induces lung cancer cell apoptosis and autophagy by repressing the STAT3/Bcl2 signaling pathway
Next, the role of the STAT3/Bcl2 signaling pathway in H2-mediated apoptosis and autophagy in lung cancer cell lines was investigated. In H1975 and A549 cells, the protein expression levels of p-STAT3 and Bcl2 were significantly decreased in a dose-dependent manner after H2 treatment (Figures 4A and B), while STAT3 overexpression abolished this effect (Figures 4C-E). Furthermore, overexpression of STAT3 in H2-treated cells inhibited lung cancer cell apoptosis and neutralized the H2-mediated increase in apoptosis (Figure 4F and G), as well as cleaved caspase 3, cleaved PARP Increased expression of , Beclin1, LC3BII/I, and p62 (all with the H2 group; Figure 4H and I). The above results indicated that H2 treatment promoted apoptosis and autophagy in lung cancer cells by inhibiting the activation of STAT3/Bcl2 pathway.
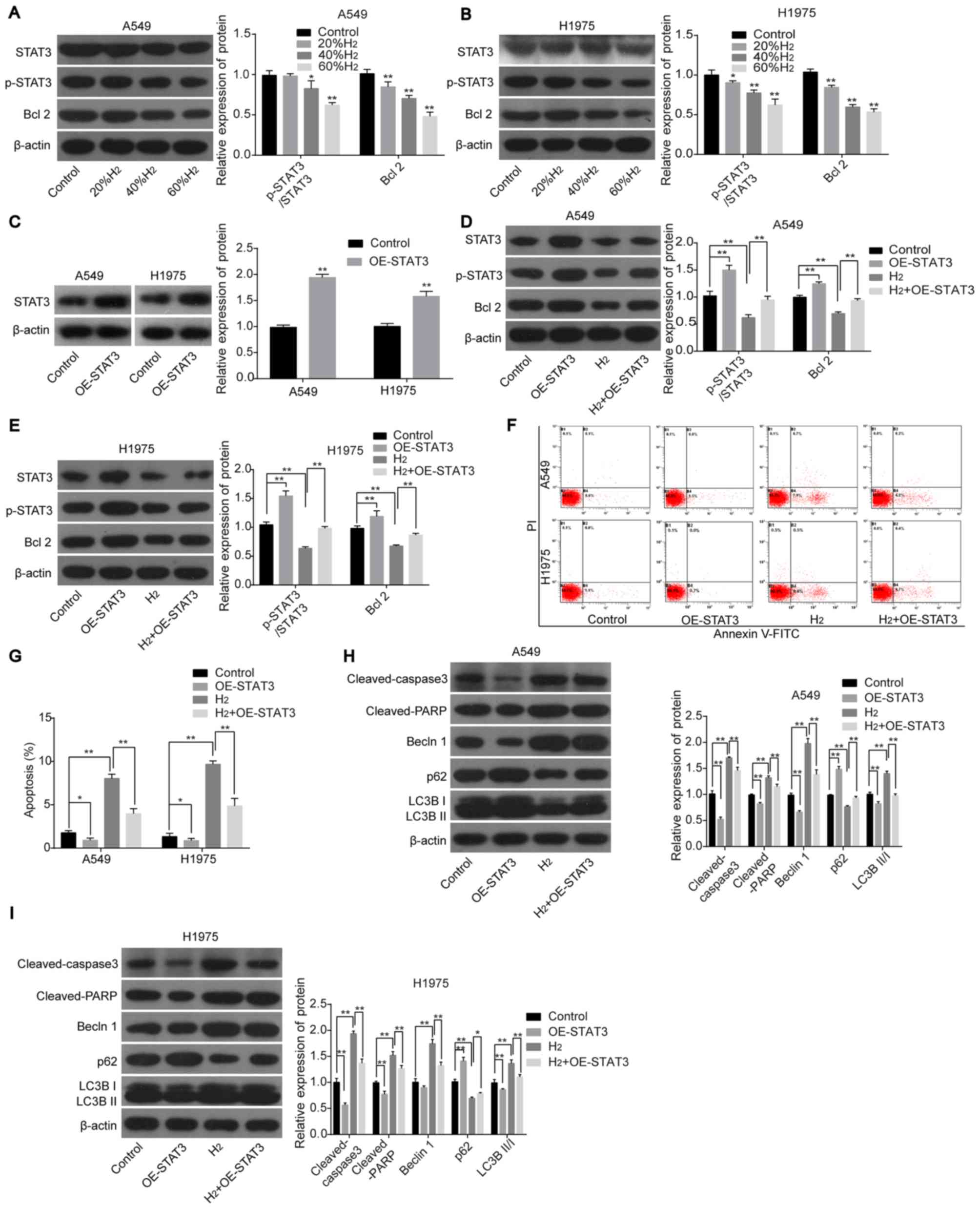
Figure 4. – H2 treatment increases lung cancer cell apoptosis and autophagy by repressing the activation of the STAT3/Bcl2 signaling pathway. The protein expression levels of STAT3, p-STAT3 and Bcl2 were detected using western blot analysis in (A) A549 and (B) H1975 cells following treatment with different concentrations of H2. (C) STAT3 expression was detected using western blot analysis following transfection with OE-STAT3 or OE-NC. The protein expression levels of STAT3, p-STAT3 and Bcl2 were detected using western blot analysis in (D) A549 and (E) H1975 cells following treatment with H2 and/or transfected with OE-STAT3. Cell apoptosis was investigated using (F) flow cytometry and the results were subsequently (G) quantified. The protein expression levels of cleaved-caspase 3, cleaved-PARP, Beclin1, p62, LC3BII and LC3BI in (H) A549 and (I) H1975 cells were detected using western blot analysis following transfection with OE-STAT3 and/or treated with H2. *P<0.05 and **P<0.01. STAT, signal transducer and activator of transcription; p-, phosphorylated; PARP, poly ADP-ribose polymerase; LC3, light chain 3; H2, hydrogen gas; OE, overexpression; NC, negative control
References
Zhao P, Jin Z, Chen Q, Yang T, Chen D, Meng J, Lu X, Gu Z and He Q: Local generation of hydrogen for enhanced photothermal therapy. Nat Commun. 9:42412018. View Article : Google Scholar : PubMed/NCBI | |
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S and Ohta S: Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007. View Article : Google Scholar : PubMed/NCBI | |
Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Nakazawa J, Kaneko K and Nakao A: Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Med Gas Res. 2:142012. View Article : Google Scholar : PubMed/NCBI | |
Chen J, Kong X, Mu F, Lu T, Du D and Xu K: Hydrogen-oxygen therapy can alleviate radiotherapy-induced hearing loss in patients with nasopharyngeal cancer. Ann Palliat Med. 8:746–751. 2019. View Article : Google Scholar : PubMed/NCBI | |
Dole M, Wilson FR and Fife WP: Hyperbaric hydrogen therapy: A possible treatment for cancer. Science. 190:152–154. 1975. View Article : Google Scholar : PubMed/NCBI | |
Wang D, Wang L, Zhang Y, Zhao Y and Chen G: Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 104:788–797. 2018. View Article : Google Scholar : PubMed/NCBI | |
Gordy C and He YW: The crosstalk between autophagy and apoptosis: Where does this lead? Protein Cell. 3:17–27. 2012. View Article : Google Scholar : PubMed/NCBI | |
Levine B and Kroemer G: Autophagy in the pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI | |
Sotthibundhu A, McDonagh K, von Kriegsheim A, Garcia-Munoz A, Klawiter A, Thompson K, Chauhan KD, Krawczyk J, McInerney V, Dockery P, et al: Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells. Stem Cell Res Ther. 7:1662016. View Article : Google Scholar : PubMed/NCBI | |
Boya P, Reggiori F and Codogno P: Emerging regulation and functions of autophagy. Nat Cell Biol. 15:713–720. 2013. View Article : Google Scholar : PubMed/NCBI | |
Levine B: Cell biology: Autophagy and cancer. Nature. 446:745–747. 2007. View Article : Google Scholar : PubMed/NCBI | |
Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, et al: Autophagy in malignant transformation and cancer progression. EMBO J. 34:856–880. 2015. View Article : Google Scholar : PubMed/NCBI | |
Wasik AM, Grabarek J, Pantovic A, Cieślar-Pobuda A, Asgari HR, Bundgaard-Nielsen C, Rafat M, Dixon IM, Ghavami S and Łos MJ: Reprogramming and carcinogenesis-parallels and distinctions. Int Rev Cell Mol Biol. 308:167–203. 2014. View Article : Google Scholar : PubMed/NCBI | |
Sridhar S, Botbol Y, Macian F and Cuervo AM: Autophagy and disease: Always two sides to a problem. J Pathol. 226:255–273. 2012. View Article : Google Scholar : PubMed/NCBI | |
Levy DE and Darnell JE Jr: Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 3:651–662. 2002. View Article : Google Scholar : PubMed/NCBI | |
Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M and Kishimoto T: Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 77:63–71. 1994. View Article : Google Scholar : PubMed/NCBI | |
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al: Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI | |
Tong M, Wang J, Jiang N, Pan H and Li D: Correlation between p-STAT3 overexpression and prognosis in lung cancer: A systematic review and meta-analysis. PLoS One. 12:e01822822017. View Article : Google Scholar : PubMed/NCBI | |
You L, Wang Z, Li H, Shou J, Jing Z, Xie J, Sui X, Pan H and Han W: The role of STAT3 in autophagy. Autophagy. 11:729–739. 2015. View Article : Google Scholar : PubMed/NCBI | |
Guo S, Luo W, Liu L, Pang X, Zhu H, Liu A, Lu J, Ma DL, Leung CH, Wang Y and Chen X: Isocryptotanshinone, a STAT3 inhibitor, induces apoptosis and pro-death autophagy in A549 lung cancer cells. J Drug Target. 24:934–942. 2016. View Article : Google Scholar : PubMed/NCBI | |
Li S, Liao R, Sheng X, Luo X, Zhang X, Wen X, Zhou J and Peng K: Hydrogen gas in cancer treatment. Front Oncol. 9:6962019. View Article : Google Scholar : PubMed/NCBI | |
Li FY, Zhu SX, Wang ZP, Wang H, Zhao Y and Chen GP: Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem Toxicol. 61:248–254. 2013. View Article : Google Scholar : PubMed/NCBI | |
Zhou P, Lin B, Wang P, Pan T, Wang S, Chen W, Cheng S and Liu S: The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J Radiat Res. 60:17–22. 2019. View Article : Google Scholar : PubMed/NCBI | |
Wu Y, Yuan M, Song J, Chen X and Yang H: Hydrogen gas from inflammation treatment to cancer therapy. ACS Nano. 13:8505–8511. 2019. View Article : Google Scholar : PubMed/NCBI | |
Chen JB, Pan ZB, Du DM, Qian W, Ma YY, Mu F and Xu KC: Hydrogen gas therapy induced shrinkage of metastatic gallbladder cancer: A case report. World J Clin Cases. 7:2065–2074. 2019. View Article : Google Scholar : PubMed/NCBI | |
Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, Buchan JR and Cho WC: Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 18:3672017. View Article : Google Scholar | |
Kroemer G, Mariño G and Levine B: Autophagy and the integrated stress response. Mol Cell. 40:280–293. 2010. View Article : Google Scholar : PubMed/NCBI | |
Dang S, Yu ZM, Zhang CY, Zheng J, Li KL, Wu Y, Qian LL, Yang ZY, Li XR, Zhang Y and Wang RX: Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Res Ther. 6:2472015. View Article : Google Scholar : PubMed/NCBI | |
Zhang M, Su L, Xiao Z and Liu X and Liu X: Methyl jasmonate induces apoptosis and pro-apoptotic autophagy via the ROS pathway in human non-small cell lung cancer. Am J Cancer Res. 6:187–199. 2016.PubMed/NCBI | |
Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, et al: Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 22:58–73. 2015. View Article : Google Scholar : PubMed/NCBI | |
White E: The role for autophagy in cancer. J Clin Invest. 125:42–46. 2015. View Article : Google Scholar : PubMed/NCBI | |
Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu P, Wang L, Xia Y, Qiao Y, Sun W, et al: Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res. 38:712019. View Article : Google Scholar : PubMed/NCBI | |
Yin C, Zhang H, Liu X, Zhang H, Zhang Y, Bai X, Wang L, Li H, Li X, Zhang S, et al: Downregulated MCOLN1 attenuates the progression of non-small-cell lung cancer by inhibiting lysosome-autophagy. Cancer Manag Res. 11:8607–8617. 2019. View Article : Google Scholar : PubMed/NCBI | |
Li Z, Wang Y, Wu L, Dong Y, Zhang J, Chen F, Xie W, Huang J and Lu N: Apurinic endonuclease 1 promotes the cisplatin resistance of lung cancer cells by inducing Parkin-mediated mitophagy. Oncol Rep. 42:2245–2254. 2019.PubMed/NCBI | |
Fan J, Ren D, Wang J, Liu X, Zhang H, Wu M and Yang G: Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 11:1262020. View Article : Google Scholar : PubMed/NCBI | |
El-Khattouti A, Selimovic D, Haikel Y and Hassan M: Crosstalk between apoptosis and autophagy: Molecular mechanisms and therapeutic strategies in cancer. J Cell Death. 6:37–55. 2013. View Article : Google Scholar : PubMed/NCBI | |
Thorburn A: Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis. 13:1–9. 2008. View Article : Google Scholar : PubMed/NCBI | |
Gupta NA, Kolachala VL, Jiang R, Abramowsky C, Shenoi A, Kosters A, Pavuluri H, Anania F and Kirk AD: Mitigation of autophagy ameliorates hepatocellular damage following ischemia-reperfusion injury in murine steatotic liver. Am J Physiol Gastrointest Liver Physiol. 307:G1088–G1099. 2014. View Article : Google Scholar : PubMed/NCBI | |
Guan P, Sun ZM, Luo LF, Zhou J, Yang S, Zhao YS, Yu FY, An JR, Wang N and Ji ES: Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 225:46–54. 2019. View Article : Google Scholar : PubMed/NCBI | |
Yan M and Yu Y, Mao X, Feng J, Wang Y, Chen H, Xie K and Yu Y: Hydrogen gas inhalation attenuates sepsis-induced liver injury in a FUNDC1-dependent manner. Int Immunopharmacol. 71:61–67. 2019. View Article : Google Scholar : PubMed/NCBI | |
Gao Y, Yang H, Chi J, Xu Q, Zhao L and Yang W, Liu W and Yang W: Hydrogen gas attenuates myocardial ischemia reperfusion injury independent of postconditioning in rats by attenuating endoplasmic reticulum stress-induced autophagy. Cell Physiol Biochem. 43:1503–1514. 2017. View Article : Google Scholar : PubMed/NCBI | |
Liu Y, Gong W, Yang ZY, Zhou XS, Gong C, Zhang TR, Wei X, Ma D, Ye F and Gao QL: Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 22:544–557. 2017. View Article : Google Scholar : PubMed/NCBI | |
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI | |
Bai X, Liu S, Yuan L, Xie Y, Li T, Wang L, Wang X, Zhang T, Qin S, Song G, et al: Hydrogen-rich saline mediates neuroprotection through the regulation of endoplasmic reticulum stress and autophagy under hypoxia-ischemia neonatal brain injury in mice. Brain Res. 1646:410–417. 2016. View Article : Google Scholar : PubMed/NCBI |
DOI: 10.3892
Published on: 20201208
Suppression of autophagy facilitates hydrogen gas‑mediated lung cancer cell apoptosis
Abstract
Our previous study found that hydrogen (H2) could effectively inhibit the progression of lung cancer; however, the underlying mechanism has not been elucidated. This study aimed to investigate the role of H2 in autophagy of lung cancer cells, and to reveal the effect of autophagy on H2-mediated apoptosis of lung cancer cells and its underlying mechanism. Expression levels of proteins associated with apoptosis and autophagy were detected using western blot analysis. Autophagy was inhibited by 3-methyladenine treatment or downregulation of Beclin1, while rapamycin was used to induce autophagy. Cell growth and apoptosis were detected using Cell Counting Kit-8 and flow cytometry, respectively. The results showed that apoptosis and autophagy were significantly enhanced in H2-treated A549 and H1975 lung cancer cell lines. However, enhanced autophagy attenuated the apoptosis-promoting effect of H2 and vice versa. Furthermore, it was found that H2 treatment induced a significant decrease in the protein expression levels of phosphorylated STAT3 and Bcl2, and overexpression of STAT3 abolished the role of H2 in promoting apoptosis and autophagy. In conclusion, this study showed that H2 could promote apoptosis and autophagy in lung cancer cells by inhibiting the activation of STAT3/Bcl2 signaling pathway, and inhibiting autophagy could enhance the role of H2 in promoting lung cancer cell apoptosis.
Results
H2 treatment induces a marked increase in apoptosis and autophagy
This study first examined the effects of H2 treatment on apoptosis and autophagy in lung cancer cells. Following H2 treatment in A549 and H1975 cells, cell viability was decreased in a time-dependent manner (Fig. 1A), while apoptotic rates were increased (Fig. 1B and C) compared to controls. The expression levels of pro-apoptotic proteins, including cleaved caspase 3 and cleaved PARP, were significantly increased in both cell lines after H2 treatment in a dose-dependent manner compared to the control group (Fig. 1D and E) (Fig. 1D and E). In addition, Beclin1 expression and LC3BII/I ratio were also significantly increased after H2 treatment, while p62 expression was significantly decreased (Figure 1D and E), also in a dose-dependent manner. These results suggest that H2 can induce apoptosis and autophagy in lung cancer cell lines.
Figure 1. – H2 treatment enhances lung cancer cell apoptosis and autophagy. After A549 and H1975 cells were treated with 20, 40 or 60% H2, the following assays were performed. (A) Cell Counting Kit-8 assay was used to investigate cell growth. (B and C) Flow cytometry assay was used to detect cell apoptosis. (D and E) The protein levels of cleaved-caspase 3, cleaved-PARP, p62, Beclin1, LC3BII and LC3BI were detected using western blot analysis. *P<0.05 and **P<0.01 vs. control. PARP, poly ADP-ribose polymerase; LC3, light chain 3; H2, hydrogen gas.
Autophagy weakens the role of H2 in promoting cell apoptosis in lung cancer cells
Subsequently, the role of autophagy in H2-induced apoptosis of lung cancer cells was examined. When A549 and H1975 cells were treated with the autophagy inducer RAPA, the protein expression levels of Beclin1 and LC3BII/I were significantly increased, while that of p62 was decreased (Figure 2A). Since 60% H2 caused significant changes in cell viability and apoptosis, 60% H2 was chosen for subsequent experiments. After RAPA treatment, the apoptosis rate (Fig. 2B) and the protein expression levels of cleaved caspase 3 and cleaved PARP (Fig. 2C and D) were significantly decreased compared with the H2 group. In contrast, the protein expression levels of Beclin1 and LC3BII/I were significantly decreased and p62 expression was increased after cells were treated with the autophagy inhibitor 3-MA (Figure 2E). Furthermore, 3-MA treatment enhanced H2-mediated increase in apoptosis rates and the protein expression levels of cleaved caspase 3 and cleaved PARP compared to the H2 group (Fig. 2G and H). These results suggest that autophagy attenuates the effect of H2 in inducing apoptosis in lung cancer cells.
Figure 2. – Evaluation of autophagy effects on H2-induced lung cancer cell apoptosis. (A) The protein expression levels of p62, Beclin1, LC3BII and LC3BI, following treatment of A549 and H1975 cells with RAPA were detected using western blot analysis. (B) Cell apoptosis was investigated using a flow cytometry assay. Western blot analysis of the expression of cleaved-caspase 3 and cleaved-PARP in (C) A549 and (D) H1975 cells treated with H2 or H2+RAPA. (E) The protein expression levels of p62, Beclin1, LC3BII and LC3BI were detected using western blot analysis following treatment of A549 and H1975 cells with 3-MA. (F) Cell apoptosis was investigated using flow cytometry. Western blot analysis of the protein expression levels of cleaved-caspase 3 and cleaved-PARP following treatment of (G) A549 and (H) H1975 cells with H2 or H2+3-MA. *P<0.05. **P<0.01. LC3, light chain 3; RAPA, rapamycin; PARP, poly ADP-ribose polymerase; PI, propidium iodide; 3-MA, 3-methyladenine; H2, hydrogen gas.
Knockdown of Beclin1 enhances the roles of H2 in promoting cell apoptosis in lung cancer cell lines
To further elucidate the role of autophagy in H2-mediated apoptosis in lung cancer cells, knockdown experiments were performed. Beclin1 expression was significantly reduced in A549 and H1975 cells transfected with siRNA targeting the human Beclin1 gene. Since si-Beclin1-3 significantly reduced the protein expression level of Beclin1 in the 3 siRNAs (Figure 3A), it was selected for subsequent experiments. After cells were transfected with si-Beclin1-3, the protein expression level of p62 was increased and the expression ratio of LC3BII:LC3BI was decreased (Figure 3B and C). Notably, H2 treatment-induced apoptosis was significantly increased when Beclin1 was silenced in A549 and H1975 cells compared with the H2 group (Fig. 3D).
Furthermore, knockdown of Beclin1 increased the protein expression levels of cleaved caspase 3 and cleaved PARP in A549 and H1975 cells after H2 treatment compared to cells treated with H2 alone (Figure 3E and F) . These results further confirmed that inhibition of autophagy could enhance the effect of H2 on apoptosis induction in lung cancer cell lines.
Figure 3. – Knockdown of Beclin1 enhances the role of H2 in promoting lung cancer cell apoptosis. (A) The knockdown efficiency of si-Beclin1 in A549 and H1975 cells was detected using western blot analysis. (B) Western blot analysis was used to detect the protein expression levels of p62, LC3BII and LC3BI in A549 and H1975 cells and the results were subsequently (C) quantified. (D) Flow cytometry assay was used to investigate cell apoptosis. The protein expression levels of cleaved-caspase 3 and cleaved-PARP were determined using western blot analysis in (E) A549 and (F) H1975 cells. *P<0.05 and **P<0.01. si, short interfering; LC3, light chain 3; PARP, poly ADP-ribose polymerase; PI, propidium iodide; H2, hydrogen gas.
H2 treatment induces lung cancer cell apoptosis and autophagy by repressing the STAT3/Bcl2 signaling pathway
Next, the role of the STAT3/Bcl2 signaling pathway in H2-mediated apoptosis and autophagy in lung cancer cell lines was investigated. In H1975 and A549 cells, the protein expression levels of p-STAT3 and Bcl2 were significantly decreased in a dose-dependent manner after H2 treatment (Figures 4A and B), while STAT3 overexpression abolished this effect (Figures 4C-E). Furthermore, overexpression of STAT3 in H2-treated cells inhibited lung cancer cell apoptosis and neutralized the H2-mediated increase in apoptosis (Figure 4F and G), as well as cleaved caspase 3, cleaved PARP Increased expression of , Beclin1, LC3BII/I, and p62 (all with the H2 group; Figure 4H and I). The above results indicated that H2 treatment promoted apoptosis and autophagy in lung cancer cells by inhibiting the activation of STAT3/Bcl2 pathway.
Figure 4. – H2 treatment increases lung cancer cell apoptosis and autophagy by repressing the activation of the STAT3/Bcl2 signaling pathway. The protein expression levels of STAT3, p-STAT3 and Bcl2 were detected using western blot analysis in (A) A549 and (B) H1975 cells following treatment with different concentrations of H2. (C) STAT3 expression was detected using western blot analysis following transfection with OE-STAT3 or OE-NC. The protein expression levels of STAT3, p-STAT3 and Bcl2 were detected using western blot analysis in (D) A549 and (E) H1975 cells following treatment with H2 and/or transfected with OE-STAT3. Cell apoptosis was investigated using (F) flow cytometry and the results were subsequently (G) quantified. The protein expression levels of cleaved-caspase 3, cleaved-PARP, Beclin1, p62, LC3BII and LC3BI in (H) A549 and (I) H1975 cells were detected using western blot analysis following transfection with OE-STAT3 and/or treated with H2. *P<0.05 and **P<0.01. STAT, signal transducer and activator of transcription; p-, phosphorylated; PARP, poly ADP-ribose polymerase; LC3, light chain 3; H2, hydrogen gas; OE, overexpression; NC, negative control
References
Zhao P, Jin Z, Chen Q, Yang T, Chen D, Meng J, Lu X, Gu Z and He Q: Local generation of hydrogen for enhanced photothermal therapy. Nat Commun. 9:42412018. View Article : Google Scholar : PubMed/NCBI
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S and Ohta S: Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007. View Article : Google Scholar : PubMed/NCBI
Ono H, Nishijima Y, Adachi N, Sakamoto M, Kudo Y, Nakazawa J, Kaneko K and Nakao A: Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers. Med Gas Res. 2:142012. View Article : Google Scholar : PubMed/NCBI
Chen J, Kong X, Mu F, Lu T, Du D and Xu K: Hydrogen-oxygen therapy can alleviate radiotherapy-induced hearing loss in patients with nasopharyngeal cancer. Ann Palliat Med. 8:746–751. 2019. View Article : Google Scholar : PubMed/NCBI
Dole M, Wilson FR and Fife WP: Hyperbaric hydrogen therapy: A possible treatment for cancer. Science. 190:152–154. 1975. View Article : Google Scholar : PubMed/NCBI
Wang D, Wang L, Zhang Y, Zhao Y and Chen G: Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 104:788–797. 2018. View Article : Google Scholar : PubMed/NCBI
Gordy C and He YW: The crosstalk between autophagy and apoptosis: Where does this lead? Protein Cell. 3:17–27. 2012. View Article : Google Scholar : PubMed/NCBI
Levine B and Kroemer G: Autophagy in the pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
Sotthibundhu A, McDonagh K, von Kriegsheim A, Garcia-Munoz A, Klawiter A, Thompson K, Chauhan KD, Krawczyk J, McInerney V, Dockery P, et al: Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells. Stem Cell Res Ther. 7:1662016. View Article : Google Scholar : PubMed/NCBI
Boya P, Reggiori F and Codogno P: Emerging regulation and functions of autophagy. Nat Cell Biol. 15:713–720. 2013. View Article : Google Scholar : PubMed/NCBI
Levine B: Cell biology: Autophagy and cancer. Nature. 446:745–747. 2007. View Article : Google Scholar : PubMed/NCBI
Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, et al: Autophagy in malignant transformation and cancer progression. EMBO J. 34:856–880. 2015. View Article : Google Scholar : PubMed/NCBI
Wasik AM, Grabarek J, Pantovic A, Cieślar-Pobuda A, Asgari HR, Bundgaard-Nielsen C, Rafat M, Dixon IM, Ghavami S and Łos MJ: Reprogramming and carcinogenesis-parallels and distinctions. Int Rev Cell Mol Biol. 308:167–203. 2014. View Article : Google Scholar : PubMed/NCBI
Sridhar S, Botbol Y, Macian F and Cuervo AM: Autophagy and disease: Always two sides to a problem. J Pathol. 226:255–273. 2012. View Article : Google Scholar : PubMed/NCBI
Levy DE and Darnell JE Jr: Stats: Transcriptional control and biological impact. Nat Rev Mol Cell Biol. 3:651–662. 2002. View Article : Google Scholar : PubMed/NCBI
Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M and Kishimoto T: Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 77:63–71. 1994. View Article : Google Scholar : PubMed/NCBI
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al: Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
Tong M, Wang J, Jiang N, Pan H and Li D: Correlation between p-STAT3 overexpression and prognosis in lung cancer: A systematic review and meta-analysis. PLoS One. 12:e01822822017. View Article : Google Scholar : PubMed/NCBI
You L, Wang Z, Li H, Shou J, Jing Z, Xie J, Sui X, Pan H and Han W: The role of STAT3 in autophagy. Autophagy. 11:729–739. 2015. View Article : Google Scholar : PubMed/NCBI
Guo S, Luo W, Liu L, Pang X, Zhu H, Liu A, Lu J, Ma DL, Leung CH, Wang Y and Chen X: Isocryptotanshinone, a STAT3 inhibitor, induces apoptosis and pro-death autophagy in A549 lung cancer cells. J Drug Target. 24:934–942. 2016. View Article : Google Scholar : PubMed/NCBI
Li S, Liao R, Sheng X, Luo X, Zhang X, Wen X, Zhou J and Peng K: Hydrogen gas in cancer treatment. Front Oncol. 9:6962019. View Article : Google Scholar : PubMed/NCBI
Li FY, Zhu SX, Wang ZP, Wang H, Zhao Y and Chen GP: Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem Toxicol. 61:248–254. 2013. View Article : Google Scholar : PubMed/NCBI
Zhou P, Lin B, Wang P, Pan T, Wang S, Chen W, Cheng S and Liu S: The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J Radiat Res. 60:17–22. 2019. View Article : Google Scholar : PubMed/NCBI
Wu Y, Yuan M, Song J, Chen X and Yang H: Hydrogen gas from inflammation treatment to cancer therapy. ACS Nano. 13:8505–8511. 2019. View Article : Google Scholar : PubMed/NCBI
Chen JB, Pan ZB, Du DM, Qian W, Ma YY, Mu F and Xu KC: Hydrogen gas therapy induced shrinkage of metastatic gallbladder cancer: A case report. World J Clin Cases. 7:2065–2074. 2019. View Article : Google Scholar : PubMed/NCBI
Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, Buchan JR and Cho WC: Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 18:3672017. View Article : Google Scholar
Kroemer G, Mariño G and Levine B: Autophagy and the integrated stress response. Mol Cell. 40:280–293. 2010. View Article : Google Scholar : PubMed/NCBI
Dang S, Yu ZM, Zhang CY, Zheng J, Li KL, Wu Y, Qian LL, Yang ZY, Li XR, Zhang Y and Wang RX: Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Res Ther. 6:2472015. View Article : Google Scholar : PubMed/NCBI
Zhang M, Su L, Xiao Z and Liu X and Liu X: Methyl jasmonate induces apoptosis and pro-apoptotic autophagy via the ROS pathway in human non-small cell lung cancer. Am J Cancer Res. 6:187–199. 2016.PubMed/NCBI
Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, et al: Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 22:58–73. 2015. View Article : Google Scholar : PubMed/NCBI
White E: The role for autophagy in cancer. J Clin Invest. 125:42–46. 2015. View Article : Google Scholar : PubMed/NCBI
Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu P, Wang L, Xia Y, Qiao Y, Sun W, et al: Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res. 38:712019. View Article : Google Scholar : PubMed/NCBI
Yin C, Zhang H, Liu X, Zhang H, Zhang Y, Bai X, Wang L, Li H, Li X, Zhang S, et al: Downregulated MCOLN1 attenuates the progression of non-small-cell lung cancer by inhibiting lysosome-autophagy. Cancer Manag Res. 11:8607–8617. 2019. View Article : Google Scholar : PubMed/NCBI
Li Z, Wang Y, Wu L, Dong Y, Zhang J, Chen F, Xie W, Huang J and Lu N: Apurinic endonuclease 1 promotes the cisplatin resistance of lung cancer cells by inducing Parkin-mediated mitophagy. Oncol Rep. 42:2245–2254. 2019.PubMed/NCBI
Fan J, Ren D, Wang J, Liu X, Zhang H, Wu M and Yang G: Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 11:1262020. View Article : Google Scholar : PubMed/NCBI
El-Khattouti A, Selimovic D, Haikel Y and Hassan M: Crosstalk between apoptosis and autophagy: Molecular mechanisms and therapeutic strategies in cancer. J Cell Death. 6:37–55. 2013. View Article : Google Scholar : PubMed/NCBI
Thorburn A: Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis. 13:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
Gupta NA, Kolachala VL, Jiang R, Abramowsky C, Shenoi A, Kosters A, Pavuluri H, Anania F and Kirk AD: Mitigation of autophagy ameliorates hepatocellular damage following ischemia-reperfusion injury in murine steatotic liver. Am J Physiol Gastrointest Liver Physiol. 307:G1088–G1099. 2014. View Article : Google Scholar : PubMed/NCBI
Guan P, Sun ZM, Luo LF, Zhou J, Yang S, Zhao YS, Yu FY, An JR, Wang N and Ji ES: Hydrogen protects against chronic intermittent hypoxia induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 225:46–54. 2019. View Article : Google Scholar : PubMed/NCBI
Yan M and Yu Y, Mao X, Feng J, Wang Y, Chen H, Xie K and Yu Y: Hydrogen gas inhalation attenuates sepsis-induced liver injury in a FUNDC1-dependent manner. Int Immunopharmacol. 71:61–67. 2019. View Article : Google Scholar : PubMed/NCBI
Gao Y, Yang H, Chi J, Xu Q, Zhao L and Yang W, Liu W and Yang W: Hydrogen gas attenuates myocardial ischemia reperfusion injury independent of postconditioning in rats by attenuating endoplasmic reticulum stress-induced autophagy. Cell Physiol Biochem. 43:1503–1514. 2017. View Article : Google Scholar : PubMed/NCBI
Liu Y, Gong W, Yang ZY, Zhou XS, Gong C, Zhang TR, Wei X, Ma D, Ye F and Gao QL: Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis. 22:544–557. 2017. View Article : Google Scholar : PubMed/NCBI
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
Bai X, Liu S, Yuan L, Xie Y, Li T, Wang L, Wang X, Zhang T, Qin S, Song G, et al: Hydrogen-rich saline mediates neuroprotection through the regulation of endoplasmic reticulum stress and autophagy under hypoxia-ischemia neonatal brain injury in mice. Brain Res. 1646:410–417. 2016. View Article : Google Scholar : PubMed/NCBI



