Introduction
Non-small-cell lung cancer (NSCLC) is the second most frequently diagnosed tumor worldwide, and is the leading cause of cancer-related deaths.1 With respect to metastasis of NSCLC, there are some preferential sites (bone, 34.3%; lung, 32.1%; brain, 28.4%; adrenal glands, 16.7%; liver, 13.4%).2 NSCLC metastasizes to the brain in 10–25% of all cases. This type of spread can lead to significant morbidity and mortality, and 5-year survival has been reported to be 3.6%.3 No significant changes in 5-year survival for patients with NSCLC metastases to the brain have been observed in the last three decades.1,4 The short-term survival of NSCLC patients with brain metastases is due to the lack of efficacious therapies. Standard chemotherapy is ineffective because of the blood–brain barrier. Radiotherapy and neurosurgery are recognized as locoregional methods;5 molecularly targeted therapies and immunotherapies are recognized as systemic treatments.6
In 2018, Wang et al7 reported on delivery of different concentrations of hydrogen gas into two culture systems for lung cancer cells (A549 and H1975). They found that the ability of cells to divide, migrate and infiltrate into tissue was inhibited significantly, and that apoptosis was accelerated. Also, lung cancer tumor-bearing mice were treated with hydrogen gas for 4 weeks, and the tumor volume was reduced by 25%. The reason for these phenomena was that hydrogen can reduce the expression of structural maintenance of chromosomes 3 (SMC3), SMC5, SMC6 in lung cancer cells.
Also in 2018, Akagi and Baba8 reported the results of hydrogen gas monotherapy for patients with stage-IV colon cancer. They found that the proportion of programmed death (PD)-1+CD8+ T-cell subsets in the blood of patients was reduced upon continuous inhalation of hydrogen, and was reduced by ~60% after several months. After 3 years of follow-up, progression-free survival and overall survival were prolonged significantly.
Here, we report a 44-year-old woman diagnosed with NSCLC and multiple metastases. After resection of an intracranial metastasis and targeted drug therapy, the outcome was good, but NSCLC metastasized (brain, cerebellum, lung, liver, bones and adrenal glands) after 28 months of treatment. In view of the literature stated above, she underwent hydrogen inhalation, and brain metastases disappeared after 4 months. Intracranial recurrence was not found after 1 year of treatment, and other metastatic sites remained stable.
Case Presentation
In November 2015, a 44-year-old woman was admitted to our hospital complaining of dizziness and instability upon standing. Through chest computed tomography (CT) and cranial magnetic resonance imaging (MRI) examination, we found multiple tumors in the left cranial cavity and right lung. After resection of an intracranial tumor, pathology studies suggested metastatic lung adenocarcinoma. Genetic testing suggested mutation of the epidermal growth factor receptor (EGFR) gene at exon 19. She started taking gefitinib tablets (Iressa™, 250 mg, once daily). The intracranial and lung tumors were controlled well until June 2017 (Figure 1).
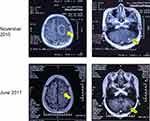 Figure 1 Post-contrast brain MR Imaging of the head before and after treatment. In November 2015, the patient was found to have lung cancer with brain (left, 4.4×3.1 cm) and cerebellar (right, 4.0×3.1 cm) metastasis. After resection and targeted drug therapy, there was no recurrence of metastases after 19 months. Solid arrow = brain metastasis; hollow arrow = cerebellar metastasis.
Figure 1 Post-contrast brain MR Imaging of the head before and after treatment. In November 2015, the patient was found to have lung cancer with brain (left, 4.4×3.1 cm) and cerebellar (right, 4.0×3.1 cm) metastasis. After resection and targeted drug therapy, there was no recurrence of metastases after 19 months. Solid arrow = brain metastasis; hollow arrow = cerebellar metastasis.
When a review was conducted in September 2017, the disease had progressed. A combination of pemetrexed and erlotinib was initiated, and the tumors were controlled again. The intracranial and lung tumors progressed again in March 2018, and the targeted treatment regimen was adjusted to icotinib hydrochloride (125 mg, t.d.s.). After 2 weeks, the patient suddenly complained of dyspnea, and difficulties in speaking. Enhanced MR imaging of the head revealed multiple nodular metastases in the left cranial cavity; the third ventricle and lateral ventricles accumulated hydrocephalus, and the largest lesion was in the frontal lobe (1.9 × 1.4 cm) (Figure 2A). Computed tomography showed that the lesion on the upper lobe of the right lung was enlarged (1.2 × 0.6 cm), and new metastases were found at the lower lobe of the right lung, mediastinum, lung hilum (1.7 × 1 cm), seventh thoracic spine and left adrenal gland. The patient refused radiotherapy and surgery of brain, signed informed consent of hydrogen therapy and agreed on the publication of the case.
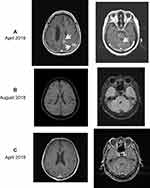
Figure 2 Post-contrast brain MRI of the head before and after hydrogen inhalation. (A) In April 2018, the patient was found to have multiple metastases (white arrows) in the brain (left, 2.5×3.0 cm and 2.4×2.8 cm for two bigger tumors) and cerebellum (right, 1.3×1.8 cm and 1.2×0.7 cm for two bigger tumors). Also, the third ventricle and lateral ventricles were expanded and had accumulated hydrocephalus. (B) After 4 months of hydrogen inhalation, all tumors visible to the naked eye had disappeared, and the shape of ventricles had returned to normal. (C) After 1 year of inhalation (April 2019), recurrence had not occurred.
From April 2018, the patient began hydrogen inhalation and received no other treatments during the time period. The hydrogen oxygen nebulizer (AMS-H-03, Asclepius Meditec, Shanghai, China) generates 3 L/min hydrogen gas by hydrocephalus electrolysis. As measured by gas chromatography, the gas generated consisted of 67% hydrogen and 33% oxygen. Using a special mask, the patient continued to inhale hydrogen for 3–6 hrs a day at rest, with no interruption even after the obvious relief of symptoms. After 4 months, most brain metastases disappeared and the amount of hydrocephalus in the third ventricle and lateral ventricles were both reduced (Figure 2B). After 1 year, all visible brain tumors had disappeared, and there were no obvious changes in metastases in the liver and lung (Figure 2C).
There were increased levels of carcinoembryonic antigen (CEA, 29.44 ng/mL), carbohydrate antigen-125 (CA125, 153 U/mL) and cytokeratin fragment antigen21-1 (CYFRA21-1, 12.1 ng/mL) before the patient started hydrogen monotherapy. Serum levels of these three markers continued to decrease after 4 months of treatment (21.6 ng/mL, 83 U/mL and 8.5 ng/mL, respectively), and were close to (but remained above) the reference range after 1 year of treatment (12.3 ng/mL, 61 U/mL and 5.9 ng/mL, respectively) (Figure 3).
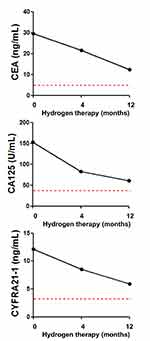
Figure 3 Detection of serum tumor markers before and after hydrogen inhalation. The red line and the lower area in each figure represent the normal range. Abbreviations: CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CYFRA21-1, cytokeratin fragment antigen 21–1.
Discussion
In general, NSCLC cells tend to metastasize to the brain, bones and adrenal glands, adenocarcinomas metastasize to the brain, and squamous cell carcinomas metastasize to bones. The exact homing mechanisms and how cancer cells communicate with this new stroma are not known.
Brain metastasis of NSCLC cells is closely related to various signaling pathways, several of which involve the participation of hydrogen gas. In 2013, Bleckmann et al9 reported that, within the Wnt pathway, T-cell factor (TCF)/lymphoid enhancer factor (LEF-1) act independently of β-catenin in human lung adenocarcinomas that metastasize to the cerebrum. In 2014, Kafka et al10 reported altered expression of dishevelled (DVL)-1, DVL-3, E-cadherin, and β-catenin in brain metastases of adenocarcinomas, which again suggested the importance of Wnt signaling. Lin et al11 were the first (in 2016) to demonstrate that hydrogen gas suppresses abnormal activation of Wnt/β-catenin signaling.
Crosstalk between EGFR and MET, the hepatocyte growth factor (HGF) receptor, has been reported in adenocarcinomas that have metastasized to the brain. This is not a direct interaction but instead signaling via activation of mitogen-activated protein kinases (MAPKs).12 In 2017, Yang et al13 found that hydrogen-containing saline can decrease phosphorylation of p38 MAPK and Smad2/3 in a rat model of cardiac fibrosis. In 2019, Guan et al14 reported that hydrogen gas can ameliorate chronic intermittent hypoxia-induced kidney injury by inhibiting oxidative stress-dependent activation of p38 and c-Jun N-terminal kinase (JNK). Similar results were demonstrated by Zhang and colleagues in experiments on the proliferation and migration of vascular smooth muscle cells.15 Han et al showed that sirtuin (SIRT)1 is highly expressed in brain metastasis,16 and several scholars have demonstrated that hydrogen can suppress SIRT1 signaling in different models.17–19 Those studies suggest that hydrogen gas has a unique therapeutic effect on the brain metastasis of NSCLC.
Several interesting studies have focused on the comparison of genomic alterations between primary lung carcinomas versus metastases to the brain and bone. The hypothesis is that there might be clonal diversity between these two. In our patient, there was a considerable difference between brain metastasis and other extrapulmonary metastases. This was most likely due to the clonal diversity of different metastatic sites, which resulted in significant differences in sensitivity to hydrogen inhalation.
As with many biomarker studies focusing on single genes/proteins, a selection bias or overinterpretation can occur. Shen et al20 found higher levels of metastasis-associated lung adenocarcinoma transcript-1 (MALAT1) in brain metastases compared with those in other extrapulmonary sites. A similar investigation searched for brain-metastasis genes and discovered a regulator of epithelial–mesenchymal transition (EMT); pre-B cell leukemia homeobox (PBX)-regulating protein-1 (PREP1) overexpression triggered EMT, whereas PREP1 downregulation inhibited EMT induction in response to transforming growth factor-β expression. PREP1 modulates sensitivity to SMAD3 and induces expression of Fos-related antigen (FRA)-1. FRA-1 and PBX1 are required for the mesenchymal changes triggered by PREP1 in lung tumor cells. PREP1-induced EMT correlates with increased lung colonization, and PREP1 accumulation has been found in human brain metastases.21 When investigating adenocarcinomas with rearrangement of activin receptor-like kinase, amplification of the fibroblast growth factor receptor (FGFR)1 gene correlated significantly with brain metastases. Although in those cases there were more visceral metastases, FGFR1 amplification in the brain metastases of adenocarcinomas were fivefold more frequent than that in the primary tumors.22
Also, C-X-C motif chemokine receptor (CXCR)4 seems to have a role in brain metastasis. CXCR4 protein has been shown to be highly overexpressed in patients with brain-specific metastasis but significantly less in NSCLC patients with metastases to other organs and in patients without metastases.23 Also, a disintegrin and metallopeptidase domain 9 (ADAM9) expression have been reported to be relatively higher in brain metastases than that in primary lung tumors. ADAM9 regulates metastasis of lung cancer cells to the brain by facilitating tissue plasminogen activator-mediated cleavage of CUB domain-containing protein-1.24
We are the first to report the efficacy of hydrogen on brain metastases from NSCLC. Our patient has survived for >1 year with hydrogen inhalation, and indicators such as serum tumor markers are improving continuously. We explored the possible reason for hydrogen to inhibited NSCLC brain metastasis in this patient (e.g. TCF/LEF-1 and DVL-1/3 of Wnt/β-catenin signaling, MAPK, and SIRT1), and proposed differences in the expression of signaling molecules between brain metastasis and other extrapulmonary metastases (e.g. MALAT1, EMT, PREP1, FGFR1, CXCR4 and ADAM9).
Hydrogen inhalation may have had a role in the treatment of our patient in different ways. For example, hydrogen exerts neuroprotective effects by reducing cyclooxygenase-2 activity25 or activating expression of anti-apoptotic protein kinase B.26 Also, hydrogen can inhibit expression of pro-apoptotic factors such as JNK and caspase-3.25,27 Hydrogen inhalation can down-regulate the expression of various pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, tumor necrosis factor-α, intracellular adhesion molecule-1, high mobility group box-1, nuclear factor-kappa B, and prostaglandin-E2.28 Whether the mechanisms mentioned above are related to the therapeutic effect of hydrogen must be confirmed by further research.
Ethics Approval and Consent for Publication
This research was approved by the research ethics committee of Fuda Cancer Hospital of Jinan University, and written informed consent for publication of the clinical details and images was obtained from the patient.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest regarding this work.


 Figure 1 Post-contrast brain MR Imaging of the head before and after treatment. In November 2015, the patient was found to have lung cancer with brain (left, 4.4×3.1 cm) and cerebellar (right, 4.0×3.1 cm) metastasis. After resection and targeted drug therapy, there was no recurrence of metastases after 19 months. Solid arrow = brain metastasis; hollow arrow = cerebellar metastasis.
Figure 1 Post-contrast brain MR Imaging of the head before and after treatment. In November 2015, the patient was found to have lung cancer with brain (left, 4.4×3.1 cm) and cerebellar (right, 4.0×3.1 cm) metastasis. After resection and targeted drug therapy, there was no recurrence of metastases after 19 months. Solid arrow = brain metastasis; hollow arrow = cerebellar metastasis.


