Hydrogen-rich water affects antioxidant activity and gut flora in soccer playersScientific Research

Effects of the long-term consumption of hydrogen-rich water on the antioxidant activity and the gut flora in female juvenile soccer players from Suzhou, China
Abstract
Results
Effects of long-term drinking of hydrogen-rich water on routine indicators of adolescent female football players
hemoglobin
After 4 weeks, the HGB of the control group decreased from 134.3±12.95 g/l to 124.00±17.75 g/l, while the HGB of the hydrogen-rich water treatment group decreased from 138.74±9.38 g/l to 129.59±8.57 g/l. After 8 weeks, the HGB of the control group increased from 124.00 ± 17.75 g/L to 131.6 ± 25.31 g/L, while the HGB of the hydrogen-rich water treatment group increased from 129.59 ± 8.57 g/L to 139.89 ± 7.02 g/L (Fig. 2A). The increasing trend and magnitude of HGB were more significant in the hydrogen-rich water treatment group (P=0.032).
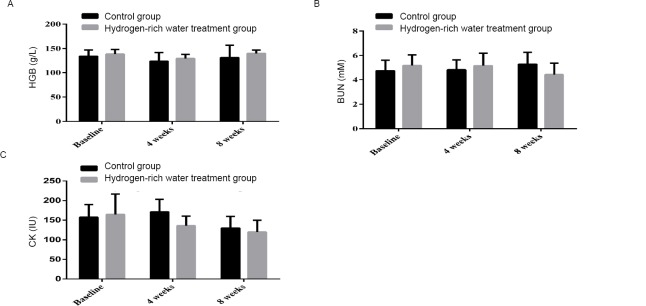
Changes in HGB, BUN and CK before and after hydrogen-rich water consumption.
Note: (A) The shift of HGB before and after hydrogen-rich water consumption; (B) The shift of BUN before and after hydrogen-rich water consumption; (C) The shift of CK before and after hydrogen-rich water consumption. HGB: Hemoglobin; BUN: blood urea nitrogen; CK: creatine kinase.
Blood urea nitrogen
After 4 weeks, the BUN level of the control group increased from 4.73 ± 0.88 to 4.83 ± 0.81 mM, while that of the hydrogen-rich water treatment group increased from 5.19 ± 0.85 to 5.17 ± 1.03 mM. After 8 weeks, BUN levels continued to increase in the control group, from 4.83 ± 0.81 to 5.29 ± 0.97 mM, while in the hydrogen-rich water-treated group, from 5.17 ± 1.03 to 4.42 ± 0.95 mM (Figure 2B). There was a more significant difference between the two groups (P = 0.887).
creatine kinase
After 4 weeks, CK in the control group increased from 157.3 ± 17.37 to 171.3 ± 31.96 IU, while that in the hydrogen-rich water treatment group increased from 149.3 ± 30.43 to 135.85 ± 24.44 IU (Fig. 2C). After 8 weeks, CK decreased from 171.3 ± 31.96 to 129.7 ± 30.05 IU in the control group and from 135.85 ± 24.44 to 119.85 ± 29.93 IU in the hydrogen-rich water treatment group (P = 0.061).
Compared to HGB and BUN, CK is more sensitive to changes in training load. These results suggest that treatment with hydrogen-rich water has some effect on increasing HGB levels in athletes’ whole blood.
Effects of long-term drinking of hydrogen-rich water on oxidation reaction indexes of adolescent female football players
Malondialdehyde
After 4 weeks, serum MDA in the control group decreased from 24.77±7.32 to 16.67±4.19 μM, while the latter decreased from 22.39±6.20 to 13.80±3.33 μM in the hydrogen-rich water treatment group. After 8 weeks, the serum MDA in the control group changed from 16.67±4.19 to 15.79±3.07 μM, and the hydrogen-rich water treatment group changed from 13.80±3.33 to 12.69±1.94 μM, and the difference between the two groups was statistically significant ( P = 0.000, Figure 3A ). ).
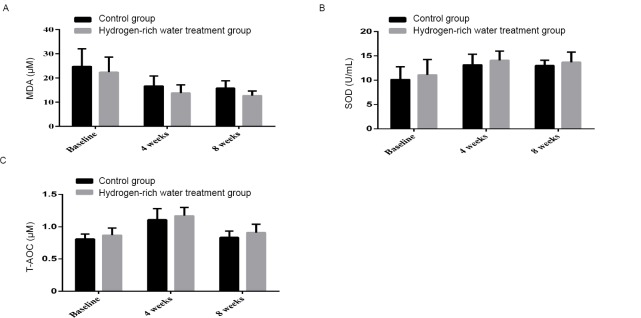
Changes in MDA, SOD and T-AOC before and after hydrogen-rich water consumption.
Note: (A) The shift of MDA before and after hydrogen-rich water consumption; (B) The shift of SOD before and after hydrogen-rich water consumption; (C) The shift of T-AOC before and after hydrogen-rich water consumption. MDA: Malondialdehyde; SOD: superoxide dismutase; T-AOC: total antioxidant capacity.
superoxide dismutase
After 4 weeks, the serum SOD level in the control group increased from 10.14±2.60 to 13.14±2.18 U/ml, and the hydrogen-rich water treatment group increased from 11.09±3.17 to 14.07±1.91 U/ml. After 8 weeks, the serum SOD level in the control group decreased from 13.14 ± 2.18 to 13.01 ± 1.08 U/ml, and the hydrogen-rich water treatment group decreased from 14.07 ± 1.91 to 13.69 ± 2.10 U/mL, with significant differences between the two groups. (P = 0.027; Figure 3B).
total antioxidant capacity
After 4 weeks, serum T-AOC in the control group increased from 0.8 ± 0.08 to 1.11 ± 0.17 μM, while serum T-AOC in the hydrogen-rich water treatment group decreased from 0.87 ± 0.11 to 1.17 ± 0.13 μM. After 8 weeks, the T-AOC of the control group changed from 1.17±0.13 to 0.84±0.09 μM, and the hydrogen-rich water treatment group changed from 1.17±0.13 to 0.9±0.13 μM, and the difference between the two groups was statistically significant (P=0.1). 0.004, Figure 3C).
These results suggest that treatment with hydrogen-rich water has an antioxidant effect.
Effects of long-term drinking of hydrogen-rich water on inflammatory indexes of young female football players
interleukin-1
After 4 weeks, the serum IL-1 level in the control group increased from 24.77±7.32 to 32.56±7.61 μM, and the hydrogen-rich water treatment group increased from 24.79±8.94 to 29.32±7.09 μM. After 8 weeks, the IL-1 level increased from 32.56±7.61 in the control group to 42.94±6.24 μM, and in the hydrogen-rich water treatment group from 29.32±7.09 μM to 34.47±6.22 μM, the difference between the two groups was statistically significant (P= 0.002, Figure 4A).
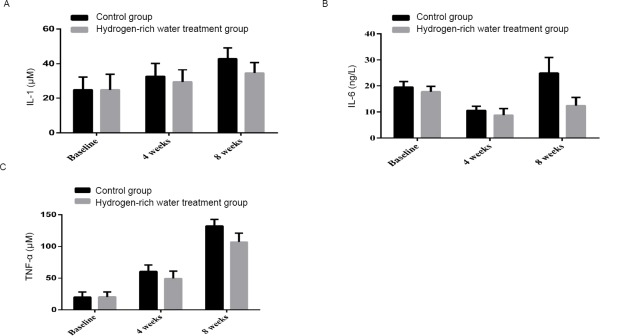
Changes in IL-1, IL-6 and TNF-α before and after hydrogen-rich water consumption.
Note: (A) The shift of IL-1 before and after hydrogen-rich water consumption; (B) The shift of IL-6 before and after hydrogen-rich water consumption; (C) The shift of TNF-α before and after hydrogen-rich water consumption. IL: Interleukin; TNF-α: tumour necrosis factor alpha.
interleukin 6
After 4 weeks, the serum IL-6 level in the control group decreased from 19.48 ± 2.16 to 10.53 ± 1.62 ng/L, and the hydrogen-rich water treatment group decreased from 17.72 ± 2.1 to 8.74 ± 2, 57 ng/L. After 8 weeks, the serum IL-6 level in the control group increased from 10.53 ± 1.62 ng/L to 24.88 ± 6.11 ng/L, while the hydrogen-rich water treatment group increased from 8.74 ± 2.57 to 12.37 ± 3.2 ng/L. There was a significant difference (P = 0.000, Figure 4B).
TNF-alpha
After 4 weeks, serum TNF-α increased from 20.04±7.99 to 60.57±10.09 μM in the control group, and increased from 20.44±7.75 to 49.46±11.59 μM in the hydrogen-rich water treatment group. After 8 weeks, the serum TNF-α in the control group increased from 60.57±10.09 to 132.24±10.46μM, and in the hydrogen-rich water treatment group from 49.46±11.59 to 107.00±13.89μM, the difference between the two groups was statistically significant (P = 0.000, Figure 4C).
These results suggest that treatment with hydrogen-rich water has an anti-inflammatory effect.
Effects of long-term drinking of hydrogen-rich water on the intestinal flora of young football players
Sort by door
In the athlete samples pretreated with hydrogen-rich water, the number of actinomycetes in the control group was higher than that in the treatment group, while the number of bacteroides in the control group was slightly lower than that in the hydrogen-rich water. – Rich Water Purification Group. In addition, the number of Clostridium in the control group was slightly higher than that in the hydrogen-rich water treatment group. However, the numbers of these bacterial groups did not differ significantly after 2 months of treatment with hydrogen-rich water.
by category
In the athlete samples pretreated with hydrogen-rich water, the number of actinomycetes in the control group was higher than that in the hydrogen-rich water treatment group, while the number of Bacteroides in the control group was slightly lower than that in the hydrogen-rich water treatment group. The numbers of Clostridium, Coccus and Erysipelas in the hydrogen-rich water treatment group were higher than those in the hydrogen-rich water treatment group. However, the numbers of these bacterial groups did not differ significantly after 2 months of treatment with hydrogen-rich water.
Sort by order
In the athlete samples pretreated with hydrogen-rich water, the number of actinomycetes in the control group was higher than that in the hydrogen-rich water treatment group, while the number of Bacteroides in the control group was slightly lower than that in the hydrogen-rich water treatment group. The numbers of Clostridium and Coccus in the hydrogen-rich water treatment group were higher than those in the hydrogen-rich water treatment group. The number of erysipelas in the control group was higher than that in the hydrogen-rich water treatment group, but the difference was not significant. However, there was no significant difference in the number of associated bacteria after 2 months of treatment with hydrogen-rich water.
Classification by family
In the athlete samples after hydrogen-rich water pretreatment, the numbers of Acidaminococcus, Bacteroidetes, Bifidobacterium, Coccus, Desulfothriaceae, Erysipelas and Ruminococcus were higher than those in the hydrogen-rich water treatment group ,The difference was statistically significant. Number of Bifidobacteriaceae, Ruminococcus, Cocci and Erysipelas. There was no difference in the number of Lachnospiraceae between the two groups. The number of Prevotella in the hydrogen-rich water treatment group was higher than that in the control group. However, the numbers of these bacterial groups did not differ significantly after 2 months of treatment with hydrogen-rich water.
Classification by genus
In the athlete samples after hydrogen-rich water pretreatment, the numbers of Bifidobacterium and Shakebacteria in the control group were higher than those in the hydrogen-rich water treatment group, but differences were observed in the number of Bifidobacterium families. Platts in the hydrogen-rich water treatment group The number of bacteria was higher than that of the control group, although the difference was not significant. The numbers of these bacterial groups were not significantly different after 2 months of treatment with hydrogen-rich water.
Effects of long-term drinking of hydrogen-rich water on the diversity and abundance of gut microbiota in adolescent female soccer players
Determine the actual number of operational taxonomic units (sobs) and the Ace, Chao, and Shannon indices, and then draw dilution curves. The recorded changes showed that the Sobs, Ace, Chao, and Shannon indices of the control group were higher than those of the hydrogen-rich water treatment group, indicating that the abundance and diversity of the intestinal flora in the control group were higher than those of the hydrogen-rich water treatment group. in the hydrogen-rich water treatment group.
After 1 month of hydrogen-rich water treatment, the indexes of sob, ace and chao in the hydrogen-rich water treatment group were higher than those in the control group. The trend was slightly reversed, indicating that the abundance of intestinal flora in the hydrogen-rich water treatment group was higher than that in the control group. At this time, the Shannon index of the treatment group was basically the same as that of the control group, indicating that treatment with hydrogen-rich water can also improve the diversity of intestinal flora.
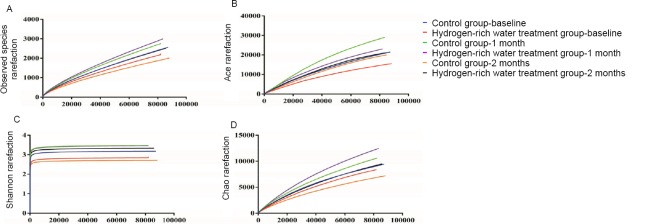
Changes in gut flora diversity and abundance before and after hydrogen-rich water consumption.
Note: (A) The shift of sobs before and after hydrogenrich water consumption; (B) The shift of ace index before and after hydrogen-rich water consumption; (C) The shift of chao index before and after hydrogen-rich water consumption; (D) The shift of shannon index before and after hydrogen-rich water consumption.
References
DOI: 10.4103
Published on: 20180812
Effects of the long-term consumption of hydrogen-rich water on the antioxidant activity and the gut flora in female juvenile soccer players from Suzhou, China
Abstract
During training and competition, a lot of physical exertion inevitably leads to fatigue. A growing body of experimental evidence confirms the link between free radical production and scavenging, fatigue and physical damage. Recently, hydrogen has been identified as a new selective antioxidant with potentially beneficial applications in exercise. This study investigated the effects of drinking hydrogen-rich water for 2 months on the gut microbiota of Suzhou youth football players. Drinking hydrogen-rich water for two months significantly reduced serum malondialdehyde, interleukin 1, interleukin 6 and tumor necrosis factor alpha as demonstrated by ELISA and 16S rDNA sequence analysis of stool samples levels of serum superoxide dismutase, total antioxidant capacity, and whole blood hemoglobin levels were then significantly increased. Furthermore, drinking hydrogen-rich water improved the diversity and abundance of the athlete’s gut microbiota. All study metrics in the control group, including Shannon, Sobs, Ace, and Chao indices, were higher than those suggested before the experiment due to hydrogen-rich water consumption, but these metrics were reversed and higher than those in the control group. Control after 2 months of intervention. Before the study, however, there were some differences in the gut microbiota composition of the two groups, while the gut microbiota composition did not change significantly during the experiment. Therefore, drinking hydrogen-enriched water for two months may exert a modulating effect on the gut microbiota of athletes based on its selective antioxidant and anti-inflammatory activities. The research protocol was approved by the Ethics Committee of Suzhou Sports School (approval number: SSS-EC150903).Results
Effects of long-term drinking of hydrogen-rich water on routine indicators of adolescent female football players
hemoglobin
After 4 weeks, the HGB of the control group decreased from 134.3±12.95 g/l to 124.00±17.75 g/l, while the HGB of the hydrogen-rich water treatment group decreased from 138.74±9.38 g/l to 129.59±8.57 g/l. After 8 weeks, the HGB of the control group increased from 124.00 ± 17.75 g/L to 131.6 ± 25.31 g/L, while the HGB of the hydrogen-rich water treatment group increased from 129.59 ± 8.57 g/L to 139.89 ± 7.02 g/L (Fig. 2A). The increasing trend and magnitude of HGB were more significant in the hydrogen-rich water treatment group (P=0.032).
Changes in HGB, BUN and CK before and after hydrogen-rich water consumption.
Note: (A) The shift of HGB before and after hydrogen-rich water consumption; (B) The shift of BUN before and after hydrogen-rich water consumption; (C) The shift of CK before and after hydrogen-rich water consumption. HGB: Hemoglobin; BUN: blood urea nitrogen; CK: creatine kinase.
Blood urea nitrogen
After 4 weeks, the BUN level of the control group increased from 4.73 ± 0.88 to 4.83 ± 0.81 mM, while that of the hydrogen-rich water treatment group increased from 5.19 ± 0.85 to 5.17 ± 1.03 mM. After 8 weeks, BUN levels continued to increase in the control group, from 4.83 ± 0.81 to 5.29 ± 0.97 mM, while in the hydrogen-rich water-treated group, from 5.17 ± 1.03 to 4.42 ± 0.95 mM (Figure 2B). There was a more significant difference between the two groups (P = 0.887).
creatine kinase
After 4 weeks, CK in the control group increased from 157.3 ± 17.37 to 171.3 ± 31.96 IU, while that in the hydrogen-rich water treatment group increased from 149.3 ± 30.43 to 135.85 ± 24.44 IU (Fig. 2C). After 8 weeks, CK decreased from 171.3 ± 31.96 to 129.7 ± 30.05 IU in the control group and from 135.85 ± 24.44 to 119.85 ± 29.93 IU in the hydrogen-rich water treatment group (P = 0.061).Compared to HGB and BUN, CK is more sensitive to changes in training load. These results suggest that treatment with hydrogen-rich water has some effect on increasing HGB levels in athletes’ whole blood.
Effects of long-term drinking of hydrogen-rich water on oxidation reaction indexes of adolescent female football players
Malondialdehyde
After 4 weeks, serum MDA in the control group decreased from 24.77±7.32 to 16.67±4.19 μM, while the latter decreased from 22.39±6.20 to 13.80±3.33 μM in the hydrogen-rich water treatment group. After 8 weeks, the serum MDA in the control group changed from 16.67±4.19 to 15.79±3.07 μM, and the hydrogen-rich water treatment group changed from 13.80±3.33 to 12.69±1.94 μM, and the difference between the two groups was statistically significant ( P = 0.000, Figure 3A ). ).
Changes in MDA, SOD and T-AOC before and after hydrogen-rich water consumption.
Note: (A) The shift of MDA before and after hydrogen-rich water consumption; (B) The shift of SOD before and after hydrogen-rich water consumption; (C) The shift of T-AOC before and after hydrogen-rich water consumption. MDA: Malondialdehyde; SOD: superoxide dismutase; T-AOC: total antioxidant capacity.
superoxide dismutase
After 4 weeks, the serum SOD level in the control group increased from 10.14±2.60 to 13.14±2.18 U/ml, and the hydrogen-rich water treatment group increased from 11.09±3.17 to 14.07±1.91 U/ml. After 8 weeks, the serum SOD level in the control group decreased from 13.14 ± 2.18 to 13.01 ± 1.08 U/ml, and the hydrogen-rich water treatment group decreased from 14.07 ± 1.91 to 13.69 ± 2.10 U/mL, with significant differences between the two groups. (P = 0.027; Figure 3B).total antioxidant capacity
After 4 weeks, serum T-AOC in the control group increased from 0.8 ± 0.08 to 1.11 ± 0.17 μM, while serum T-AOC in the hydrogen-rich water treatment group decreased from 0.87 ± 0.11 to 1.17 ± 0.13 μM. After 8 weeks, the T-AOC of the control group changed from 1.17±0.13 to 0.84±0.09 μM, and the hydrogen-rich water treatment group changed from 1.17±0.13 to 0.9±0.13 μM, and the difference between the two groups was statistically significant (P=0.1). 0.004, Figure 3C).These results suggest that treatment with hydrogen-rich water has an antioxidant effect.
Effects of long-term drinking of hydrogen-rich water on inflammatory indexes of young female football players
interleukin-1
After 4 weeks, the serum IL-1 level in the control group increased from 24.77±7.32 to 32.56±7.61 μM, and the hydrogen-rich water treatment group increased from 24.79±8.94 to 29.32±7.09 μM. After 8 weeks, the IL-1 level increased from 32.56±7.61 in the control group to 42.94±6.24 μM, and in the hydrogen-rich water treatment group from 29.32±7.09 μM to 34.47±6.22 μM, the difference between the two groups was statistically significant (P= 0.002, Figure 4A).
Changes in IL-1, IL-6 and TNF-α before and after hydrogen-rich water consumption.
Note: (A) The shift of IL-1 before and after hydrogen-rich water consumption; (B) The shift of IL-6 before and after hydrogen-rich water consumption; (C) The shift of TNF-α before and after hydrogen-rich water consumption. IL: Interleukin; TNF-α: tumour necrosis factor alpha.
interleukin 6
After 4 weeks, the serum IL-6 level in the control group decreased from 19.48 ± 2.16 to 10.53 ± 1.62 ng/L, and the hydrogen-rich water treatment group decreased from 17.72 ± 2.1 to 8.74 ± 2, 57 ng/L. After 8 weeks, the serum IL-6 level in the control group increased from 10.53 ± 1.62 ng/L to 24.88 ± 6.11 ng/L, while the hydrogen-rich water treatment group increased from 8.74 ± 2.57 to 12.37 ± 3.2 ng/L. There was a significant difference (P = 0.000, Figure 4B).TNF-alpha
After 4 weeks, serum TNF-α increased from 20.04±7.99 to 60.57±10.09 μM in the control group, and increased from 20.44±7.75 to 49.46±11.59 μM in the hydrogen-rich water treatment group. After 8 weeks, the serum TNF-α in the control group increased from 60.57±10.09 to 132.24±10.46μM, and in the hydrogen-rich water treatment group from 49.46±11.59 to 107.00±13.89μM, the difference between the two groups was statistically significant (P = 0.000, Figure 4C).These results suggest that treatment with hydrogen-rich water has an anti-inflammatory effect.
Effects of long-term drinking of hydrogen-rich water on the intestinal flora of young football players
Sort by door
In the athlete samples pretreated with hydrogen-rich water, the number of actinomycetes in the control group was higher than that in the treatment group, while the number of bacteroides in the control group was slightly lower than that in the hydrogen-rich water. – Rich Water Purification Group. In addition, the number of Clostridium in the control group was slightly higher than that in the hydrogen-rich water treatment group. However, the numbers of these bacterial groups did not differ significantly after 2 months of treatment with hydrogen-rich water.by category
In the athlete samples pretreated with hydrogen-rich water, the number of actinomycetes in the control group was higher than that in the hydrogen-rich water treatment group, while the number of Bacteroides in the control group was slightly lower than that in the hydrogen-rich water treatment group. The numbers of Clostridium, Coccus and Erysipelas in the hydrogen-rich water treatment group were higher than those in the hydrogen-rich water treatment group. However, the numbers of these bacterial groups did not differ significantly after 2 months of treatment with hydrogen-rich water.
Sort by order
In the athlete samples pretreated with hydrogen-rich water, the number of actinomycetes in the control group was higher than that in the hydrogen-rich water treatment group, while the number of Bacteroides in the control group was slightly lower than that in the hydrogen-rich water treatment group. The numbers of Clostridium and Coccus in the hydrogen-rich water treatment group were higher than those in the hydrogen-rich water treatment group. The number of erysipelas in the control group was higher than that in the hydrogen-rich water treatment group, but the difference was not significant. However, there was no significant difference in the number of associated bacteria after 2 months of treatment with hydrogen-rich water.Classification by family
In the athlete samples after hydrogen-rich water pretreatment, the numbers of Acidaminococcus, Bacteroidetes, Bifidobacterium, Coccus, Desulfothriaceae, Erysipelas and Ruminococcus were higher than those in the hydrogen-rich water treatment group ,The difference was statistically significant. Number of Bifidobacteriaceae, Ruminococcus, Cocci and Erysipelas. There was no difference in the number of Lachnospiraceae between the two groups. The number of Prevotella in the hydrogen-rich water treatment group was higher than that in the control group. However, the numbers of these bacterial groups did not differ significantly after 2 months of treatment with hydrogen-rich water.
Classification by genus
In the athlete samples after hydrogen-rich water pretreatment, the numbers of Bifidobacterium and Shakebacteria in the control group were higher than those in the hydrogen-rich water treatment group, but differences were observed in the number of Bifidobacterium families. Platts in the hydrogen-rich water treatment group The number of bacteria was higher than that of the control group, although the difference was not significant. The numbers of these bacterial groups were not significantly different after 2 months of treatment with hydrogen-rich water.
Effects of long-term drinking of hydrogen-rich water on the diversity and abundance of gut microbiota in adolescent female soccer players
Determine the actual number of operational taxonomic units (sobs) and the Ace, Chao, and Shannon indices, and then draw dilution curves. The recorded changes showed that the Sobs, Ace, Chao, and Shannon indices of the control group were higher than those of the hydrogen-rich water treatment group, indicating that the abundance and diversity of the intestinal flora in the control group were higher than those of the hydrogen-rich water treatment group. in the hydrogen-rich water treatment group.
After 1 month of hydrogen-rich water treatment, the indexes of sob, ace and chao in the hydrogen-rich water treatment group were higher than those in the control group. The trend was slightly reversed, indicating that the abundance of intestinal flora in the hydrogen-rich water treatment group was higher than that in the control group. At this time, the Shannon index of the treatment group was basically the same as that of the control group, indicating that treatment with hydrogen-rich water can also improve the diversity of intestinal flora.
Changes in gut flora diversity and abundance before and after hydrogen-rich water consumption.
Note: (A) The shift of sobs before and after hydrogenrich water consumption; (B) The shift of ace index before and after hydrogen-rich water consumption; (C) The shift of chao index before and after hydrogen-rich water consumption; (D) The shift of shannon index before and after hydrogen-rich water consumption.
References
1. Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol Respir Environ Exerc Physiol. 1978;45:927–932. [PubMed] [Google Scholar]2. Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. [PubMed] [Google Scholar]3. Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic Biol Med. 2011;51:942–950. [PubMed] [Google Scholar]4. Cobley JN, McHardy H, Morton JP, Nikolaidis MG, Close GL. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free Radic Biol Med. 2015;84:65–76. [PubMed] [Google Scholar]5. Pingitore A, Pereira Lima GP, Mastorci F, Quinones A, Iervasi G, Vassalle C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31:916–922. [PubMed] [Google Scholar]6. Slattery K, Bentley D, Coutts AJ. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. 2015;45:453–471. [PubMed] [Google Scholar]7. Mankowski RT, Anton SD, Buford TW, Leeuwenburgh C. Dietary antioxidants as modifiers of physiologic adaptations to exercise. Med Sci Sports Exerc. 2015;47:1857–1868. [PMC free article] [PubMed] [Google Scholar]8. McAnulty LS, Miller LE, Hosick PA, Utter AC, Quindry JC, McAnulty SR. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl Physiol Nutr Metab. 2013;38:760–765. [PubMed] [Google Scholar]9. Carrera-Quintanar L, Funes L, Vicente-Salar N, et al. Effect of polyphenol supplements on redox status of blood cells: a randomized controlled exercise training trial. Eur J Nutr. 2015;54:1081–1093. [PubMed] [Google Scholar]10. Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. [PubMed] [Google Scholar]11. Zhao L. Genomics: The tale of our other genome. Nature. 2010;465:879–880. [PubMed] [Google Scholar]12. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. [PubMed] [Google Scholar]13. Espley RV, Butts CA, Laing WA, et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J Nutr. 2014;144:146–154. [PubMed] [Google Scholar]14. Placha I, Chrastinova L, Laukova A, et al. Effect of thyme oil on small intestine integrity and antioxidant status, phagocytic activity and gastrointestininal microbita in rabbits. Acta Vet Hung. 2013;61:197–208. [PubMed] [Google Scholar]15. Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010;44:971–982. [PubMed] [Google Scholar]16. Noda M, Fujita K, Ohsawa I, Ito M, Ohno K. Multiple effects of Mecular hydrogen and its distinct mechanism. J Neurol Disord. 2014;2:1–8. [Google Scholar]17. Ostojic SM, Stojanovic MD. Hydrogen-rich water affected blood alkalinity in physically active men. Res Sports Med. 2014;22:49–60. [PubMed] [Google Scholar]18. Ostojic SM. Molecular hydrogen in sports medicine: new therapeutic perspectives. Int J Sports Med. 2015;36:273–279. [PubMed] [Google Scholar]19. Tsubone H, Hanafusa M, Endo M, et al. Effect of treadmill exercise and hydrogen-rich water intake on serum oxidative and anti-oxidative metabolites in serum of thoroughbred horses. J Equine Sci. 2013;24:1–8. [PMC free article] [PubMed] [Google Scholar]20. Aoki K, Nakao A, Adachi T, Matsui Y, Miyakawa S. Pilot study: Effects of drinking hydrogen-rich water on muscle fatigue caused by acute exercise in elite athletes. Med Gas Res. 2012;2:12. [PMC free article] [PubMed] [Google Scholar]21. Li A, Zhang L, Zhou J, Sun X. Effects of supplementation of hydrogen-rich water on the oxidative stress-induced damage in skeletal muscle after acute exhaustive exercise. Zhongguo Yundong Yixue. 2011;30:452–455. [Google Scholar]22. Zhao YY, Zhang L. Master Dissertation. Suzhou: Schoow University; 2014. The influence of drinking hydrogen-rich water at different phase and high-intensity exercise to swimming athletes in free radical metabolism. [Google Scholar]23. Hu J, Zhang L. Master Dissertation. Suzhou: Schoow University; 2014. A comparative research of the influence of different antioxidants on antioxidant system about the short distance swimmer. [Google Scholar]24. Li C, Li CX, Pang L, Wu L. Hydrogen-rich water on oxidative stress injury in rat skeletal muscle after exhaustive exercise was repeated. Taishan Yixueyuan Xuebao. 2015;36:371–375. [Google Scholar]25. Wang L, Liu ZQ, Hou YL, Ge YJ. Hydrogen-rich water inhibits mitochondrial oxidative stress and inflammation in the skeletal muscle after eccentric exercise. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:4682–4687. [Google Scholar]26. Ji LL, Zhang Y. Antioxidant and anti-inflammatory effects of exercise: role of redox signaling. Free Radic Res. 2014;48:3–11. [PubMed] [Google Scholar]27. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. [PubMed] [Google Scholar]28. Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241–1249. [PubMed] [Google Scholar]29. Wang B, Sun J, Li X, et al. Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr Res. 2013;33:971–981. [PubMed] [Google Scholar]30. Jose Pozuelo M, Agis-Torres A, Hervert-Hernandez D, et al. Grape antioxidant dietary fiber stimulates Lactobacillus growth in rat cecum. J Food Sci. 2012;77:H59–H62. [PubMed] [Google Scholar]31. Kasaikina MV, Kravtsova MA, Lee BC, et al. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011;25:2492–2499. [PMC free article] [PubMed] [Google Scholar]32. Jakobsdottir G, Blanco N, Xu J, et al. Formation of short-chain Fatty acids, excretion of anthocyanins, and microbial diversity in rats fed blackcurrants, blackberries, and raspberries. J Nutr Metab. 2013;2013:202534. [PMC free article] [PubMed] [Google Scholar]33. Neyrinck AM, Van Hee VF, Bindels LB, De Backer F, Cani PD, Delzenne NM. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br J Nutr. 2013;109:802–809. [PubMed] [Google Scholar]34. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. [PubMed] [Google Scholar]35. Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. [PubMed] [Google Scholar]36. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. [PubMed] [Google Scholar]37. Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. [PubMed] [Google Scholar]



