Hydrogen treatment of skin diseasesScientific Research
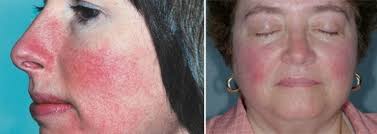
Treatment with hydrogen (H2) of acute erythematous skin diseases
Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers
4 patients with acute erythematous skin diseases with fever and/or pain were treated by intravenous administration of hydrogen-enriched IV fluid. We also added data from two volunteers to evaluate the mode of delivery of H 2 to the skin to assess the feasibility of H 2 treatment for this type of skin disease. All four patients received intravenous administration of 500 ml of H2-enriched fluid over 30 minutes for more than 3 days, except for one patient only once.From two volunteers (one for intravenous H2 and the other for inhaled H2), blood samples were drawn sequentially and air samples were collected from a heavy plastic bag covering the leg before, during, and after H2 administration. These samples were checked for H2 concentration immediately by gas chromatography. Numerous physiological parameters and blood chemistry data were also collected. These 4 patients’ erythema and associated symptoms improved significantly after H2 treatment and did not recur. Administration of H2 does not change physiological parameters and does not cause deterioration of blood chemistry.
Improvement in acute erythematous skin disease followed administration of hydrogen-enriched H2 fluid without compromising safety. The hydrogen H2 delivery study of two volunteers suggested initial direct delivery and additional sustained delivery, possibly from a slowly depleting reservoir in the surface skin.
Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers
DOI: 10.1186
Published on: 20121205
Treatment with hydrogen (H2) of acute erythematous skin diseases
Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers
4 patients with acute erythematous skin diseases with fever and/or pain were treated by intravenous administration of hydrogen-enriched IV fluid. We also added data from two volunteers to evaluate the mode of delivery of H 2 to the skin to assess the feasibility of H 2 treatment for this type of skin disease. All four patients received intravenous administration of 500 ml of H2-enriched fluid over 30 minutes for more than 3 days, except for one patient only once.From two volunteers (one for intravenous H2 and the other for inhaled H2), blood samples were drawn sequentially and air samples were collected from a heavy plastic bag covering the leg before, during, and after H2 administration. These samples were checked for H2 concentration immediately by gas chromatography. Numerous physiological parameters and blood chemistry data were also collected. These 4 patients’ erythema and associated symptoms improved significantly after H2 treatment and did not recur. Administration of H2 does not change physiological parameters and does not cause deterioration of blood chemistry.
The concentration of H2 in the blood of the volunteers increased rapidly with H2 inhalation and slowly decreased with the cessation of H2, especially in venous blood, whereas the H2 concentration in air from the leg surface showed much slower changes even after H2 inhalation ceased. , at least during sampling.
Improvement in acute erythematous skin disease followed administration of hydrogen-enriched H2 fluid without compromising safety. The hydrogen H2 delivery study of two volunteers suggested initial direct delivery and additional sustained delivery, possibly from a slowly depleting reservoir in the surface skin.Published online 2012 May 20. doi: 10.1186/2045-9912-2-14PMCID: PMC3407032PMID: 22607973Hydrogen(H2) treatment for acute erythymatous skin diseases. A report of 4 patients with safety data and a non-controlled feasibility study with H2 concentration measurement on two volunteers


 #1
#1 
