Hydrogen-rich water protect liver of cancer patients with chemotherapyScientific Research
Protective effect of hydrogen-rich water on liver function of colorectal cancer patients treated with mFOLFOX6 chemotherapy
Abstract
This study aimed to investigate the protective effect of hydrogen-rich water on liver function in colorectal cancer (CRC) patients receiving mFOLFOX6 chemotherapy. A controlled, randomized, single-blind clinical study was designed. Between June 2010 and February 2016, 152 CRC patients were recruited from the Oncology Department of Taishan Hospital (Taian, China), of whom 146 met the inclusion criteria. Subsequently, 144 patients were randomized to treatment (n=80) and placebo (n=64). At the end of the study, 76 patients in the hydrogen treatment group and 60 patients in the placebo group were included in the final analysis. Changes in liver function after chemotherapy have been observed, such as changes in levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, indirect bilirubin (IBIL), and direct bilirubin . The adverse effects of mFOLFOX6 chemotherapy on liver function were mainly manifested as increased levels of ALT, AST and IBIL. There was no significant difference in liver function before and after treatment in the hydrogen-rich water group, while the levels of ALT, AST and IBIL in the placebo group were significantly increased. Therefore, hydrogen-rich water appears to ameliorate mFOLFOX6-induced liver injury.
Results
Patients
As shown in Table I, a total of 136 patients were analyzed. There were no significant stratified differences in each factor (age, gender, ECOG-PS, smoking history, drinking history, liver metastases, chemotherapy cycle) between the two groups (P>0.05); therefore, randomization was considered to be baseline balanced.
Effects of mFOLFOX6 on liver function
As shown in Table III, the effects of mFOLFOX6 chemotherapy on the liver were mainly manifested by increased levels of ALT, AST, and IBIL. After chemotherapy, 46, 40, 11, 6, and 17 cases had abnormal ALT, AST, ALP, DBIL, and IBIL, accounting for 33.82, 29.41, 8.09, 4, 41, and 12.50%, respectively.
Table III.
Types of hepatic damage in patients exhibiting mFOLFOX6-induced liver injury.
| Abnormal marker, patient no. (%) | ||||
|---|---|---|---|---|
| AST | ALT | ALP | DBIL | IBIL |
| 46 (33.82) | 40 (29.41) | 11 (8.09) | 6 (4.41) | 17 (12.50) |
mFOLFOX6, 5-FU, oxaliplatin and calcium folinate; ALT, alanine aminotransferase; AST, aspartate transaminase; ALP, alkaline phosphatase; DBIL, direct bilirubin; IBIL, indirect bilirubin.
Comparison of liver damage between hydrogen-rich water and placebo groups
The probability and degree of chemotherapy-induced liver damage in the hydrogen-rich water group were lower compared with those in the placebo group. The comparison of hepatic injury following chemotherapy between the two groups was performed with the use of the rank-sum test. As shown in Table IV, the patients in the hydrogen-rich water group had a lower probability and degree of hepatic damage compared with those in the placebo group (P<0.05).
Table IV.
Comparison of hepatic damage between the hydrogen-rich water and control groups.
| Groups | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Mean-rank |
|---|---|---|---|---|---|---|
| Hydrogen-rich water | 54 | 14 | 4 | 4 | 0 | 58.13 |
| Control | 24 | 16 | 10 | 8 | 2 | 81.63 |
| P-value | 0.00 |
Comparison of liver function tests before and after treatment between the two groups
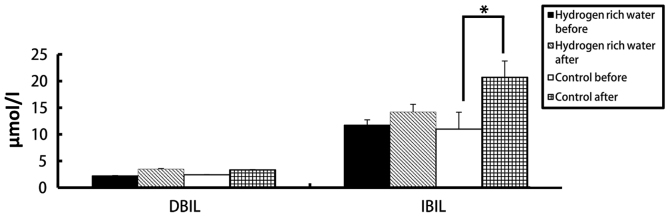
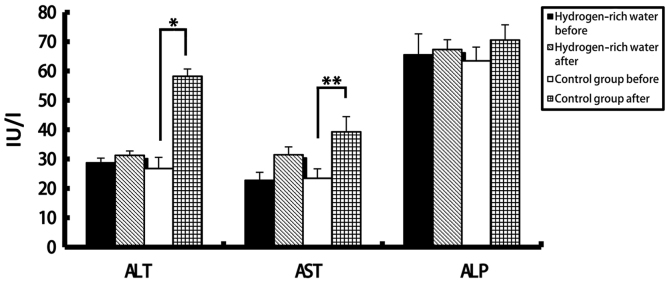
There was no significant difference in the levels of ALT, AST, ALP, DBIL and IBIL in the hydrogen-rich water group before and after chemotherapy. However, there were statistically significant differences in ALT, AST, and DBIL values in the control group before and after chemotherapy (see Figures 2 and 3). The ALT levels in the hydrogen-rich water group before and after chemotherapy were 28.72±1.6 and 31.28±1.47 IU/L, respectively; the difference was not statistically significant (p=0.46). The ALT levels in the control group before and after chemotherapy were 26.78±3.8 and 58.22±2.46 IU/L, respectively; the difference was statistically significant (P=0.04). The AST values in the hydrogen-rich water group before and after chemotherapy were 22.74±2.74 and 23.43±2.66 IU/L, respectively; the difference was not statistically significant (p=0.67).AST in the control group before and after chemotherapy was 23.43 ± 3.24 and 39.28 ± 5.17 IU/L, respectively; the difference was statistically significant (P=0.032).
Comparison of changes in ALT, AST and ALP levels before and after chemotherapy between the two groups. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase. *P=0.04; **P=0.032.
Comparison of changes in DBIL and IBIL levels before and after chemotherapy between the two groups. *P=0.046.
References
Yang Q, Ji G, Pan R, et al. Protective effect of hydrogen-rich water on liver function of colorectal cancer patients treated with mFOLFOX6 chemotherapy. Mol Clin Oncol. 2017 Nov;7(5):891-896.
DOI: 10.3892
Published on: 20170711
Protective effect of hydrogen-rich water on liver function of colorectal cancer patients treated with mFOLFOX6 chemotherapy
Abstract
This study aimed to investigate the protective effect of hydrogen-rich water on liver function in colorectal cancer (CRC) patients receiving mFOLFOX6 chemotherapy. A controlled, randomized, single-blind clinical study was designed. Between June 2010 and February 2016, 152 CRC patients were recruited from the Oncology Department of Taishan Hospital (Taian, China), of whom 146 met the inclusion criteria. Subsequently, 144 patients were randomized to treatment (n=80) and placebo (n=64). At the end of the study, 76 patients in the hydrogen treatment group and 60 patients in the placebo group were included in the final analysis. Changes in liver function after chemotherapy have been observed, such as changes in levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, indirect bilirubin (IBIL), and direct bilirubin . The adverse effects of mFOLFOX6 chemotherapy on liver function were mainly manifested as increased levels of ALT, AST and IBIL. There was no significant difference in liver function before and after treatment in the hydrogen-rich water group, while the levels of ALT, AST and IBIL in the placebo group were significantly increased. Therefore, hydrogen-rich water appears to ameliorate mFOLFOX6-induced liver injury.
Results
Patients
As shown in Table I, a total of 136 patients were analyzed. There were no significant stratified differences in each factor (age, gender, ECOG-PS, smoking history, drinking history, liver metastases, chemotherapy cycle) between the two groups (P>0.05); therefore, randomization was considered to be baseline balanced.
Effects of mFOLFOX6 on liver function
As shown in Table III, the effects of mFOLFOX6 chemotherapy on the liver were mainly manifested by increased levels of ALT, AST, and IBIL. After chemotherapy, 46, 40, 11, 6, and 17 cases had abnormal ALT, AST, ALP, DBIL, and IBIL, accounting for 33.82, 29.41, 8.09, 4, 41, and 12.50%, respectively.
Table III.
Types of hepatic damage in patients exhibiting mFOLFOX6-induced liver injury.
Abnormal marker, patient no. (%) AST ALT ALP DBIL IBIL 46 (33.82) 40 (29.41) 11 (8.09) 6 (4.41) 17 (12.50) mFOLFOX6, 5-FU, oxaliplatin and calcium folinate; ALT, alanine aminotransferase; AST, aspartate transaminase; ALP, alkaline phosphatase; DBIL, direct bilirubin; IBIL, indirect bilirubin.
Comparison of liver damage between hydrogen-rich water and placebo groups
The probability and degree of chemotherapy-induced liver damage in the hydrogen-rich water group were lower compared with those in the placebo group. The comparison of hepatic injury following chemotherapy between the two groups was performed with the use of the rank-sum test. As shown in Table IV, the patients in the hydrogen-rich water group had a lower probability and degree of hepatic damage compared with those in the placebo group (P<0.05).
Table IV.
Comparison of hepatic damage between the hydrogen-rich water and control groups.
Groups Grade 0 Grade 1 Grade 2 Grade 3 Grade 4 Mean-rank Hydrogen-rich water 54 14 4 4 0 58.13 Control 24 16 10 8 2 81.63 P-value 0.00
Comparison of liver function tests before and after treatment between the two groups
There was no significant difference in the levels of ALT, AST, ALP, DBIL and IBIL in the hydrogen-rich water group before and after chemotherapy. However, there were statistically significant differences in ALT, AST, and DBIL values in the control group before and after chemotherapy (see Figures 2 and 3). The ALT levels in the hydrogen-rich water group before and after chemotherapy were 28.72±1.6 and 31.28±1.47 IU/L, respectively; the difference was not statistically significant (p=0.46). The ALT levels in the control group before and after chemotherapy were 26.78±3.8 and 58.22±2.46 IU/L, respectively; the difference was statistically significant (P=0.04). The AST values in the hydrogen-rich water group before and after chemotherapy were 22.74±2.74 and 23.43±2.66 IU/L, respectively; the difference was not statistically significant (p=0.67).AST in the control group before and after chemotherapy was 23.43 ± 3.24 and 39.28 ± 5.17 IU/L, respectively; the difference was statistically significant (P=0.032).
Comparison of changes in ALT, AST and ALP levels before and after chemotherapy between the two groups. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase. *P=0.04; **P=0.032.
Comparison of changes in DBIL and IBIL levels before and after chemotherapy between the two groups. *P=0.046.
References
1. Christova TY, Gorneva GA, Taxirov SI, Duridanova DB, Setchenska MS. Effect of cisplatin and cobalt chloride on antioxidant enzymes in the livers of Lewis lung carcinoma bearing mice: Protective role of heme oxygenase. Toxicol Lett. 2003;138:235–242. doi: 10.1016/S0378-4274(02)00416-2. [PubMed] [CrossRef] [Google Scholar]2. Koc A, Duru M, Ciralik H, Akcan R, Sogut S. Protective agent, erdosteine, against cisplatin-induced hepatic oxidant injury in rats. Mol Cell Biochem. 2005;278:79–84. doi: 10.1007/s11010-005-6630-z. [PubMed] [CrossRef] [Google Scholar]3. Robinson K, Lambiase L, Li J, Monteiro C, Schiff M. Fatal cholestatic liver failure associated with gemcitabine therapy. Dig Dis Sci. 2003;48:1804–1808. doi: 10.1023/A:1025415616592. [PubMed] [CrossRef] [Google Scholar]4. Bibeau F, Azria D, Chateau MC, Borrelly C, Ychou M, Quenet F, Rouanet P. Vascular hepatic injury following neoadjuvant treatment for a cardial adenocarcinoma. Gastroenterol Clin Biol. 2006;30:611–613. doi: 10.1016/S0399-8320(06)73237-7. (In French) [PubMed] [CrossRef] [Google Scholar]5. Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010;44:971–282. doi: 10.3109/10715762.2010.500328. [PubMed] [CrossRef] [Google Scholar]6. Cardinal JS, Zhan J, Wang Y, Sugimoto R, Tsung A, McCurry KR, Billiar TR, Nakao A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010;77:101–109. doi: 10.1038/ki.2009.421. [PubMed] [CrossRef] [Google Scholar]7. Mao YF, Zheng XF, Cai JM, You XM, Deng XM, Zhang JH, Jiang L, Sun XJ. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2009;381:602–605. doi: 10.1016/j.bbrc.2009.02.105. [PubMed] [CrossRef] [Google Scholar]8. National Comprehensive Cancer Network: (NCCN) Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Colon Cancer. 2012 2011 Aug 30; Version 1. Accessed. [Google Scholar]9. Fong Y, Bentrem DJ. CASH (chemotherapy-associated steatohepatitis) costs. Ann Surg. 2006;243:8–9. doi: 10.1097/01.sla.0000193599.57858.9b. [PMC free article] [PubMed] [CrossRef] [Google Scholar]10. Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013;2013:815105. doi: 10.1155/2013/815105. [PMC free article] [PubMed] [CrossRef] [Google Scholar]11. Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: Impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860–868. doi: 10.1007/s11605-007-0149-4. [PubMed] [CrossRef] [Google Scholar]12. Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [PubMed] [CrossRef] [Google Scholar]13. Tamandl D, Klinger M, Eipeldauer S, Herberger B, Kaczirek K, Gruenberger B, Gruenberger T. Sinusoidal obstruction syndrome impairs long-term outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:421–430. doi: 10.1245/s10434-010-1317-4. [PubMed] [CrossRef] [Google Scholar]14. Laurent A, Nicco C, Van Nhieu Tran J, Borderie D, Chéreau C, Conti F, Jaffray P, Soubrane O, Calmus Y, Weill B, Batteux F. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology. 2004;39:1277–1285. doi: 10.1002/hep.20177. [PubMed] [CrossRef] [Google Scholar]15. Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21:57–69. doi: 10.1055/s-2001-12929. [PubMed] [CrossRef] [Google Scholar]16. Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane O, Chaussade S, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [PubMed] [CrossRef] [Google Scholar]17. Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P, Jaeck D. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [PubMed] [CrossRef] [Google Scholar]18. Dyson JK, McPherson S, Anstee QM. Non-alcoholic fatty liver disease: Non-invasive investigation and risk stratification. J Clin Pathol. 2013;66:1033–1045. doi: 10.1136/jclinpath-2013-201620. [PubMed] [CrossRef] [Google Scholar]19. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [PubMed] [CrossRef] [Google Scholar]20. Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30–35. doi: 10.1016/j.bbrc.2008.05.165. [PubMed] [CrossRef] [Google Scholar]21. Buchholz BM, Kaczorowski DJ, Sugimoto R, Yang R, Wang Y, Billiar TR, McCurry KR, Bauer AJ, Nakao A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8:2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [PubMed] [CrossRef] [Google Scholar]22. Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang JH, Denoble PJ, Tao H, Sun X. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Exp Biol Med (Maywood) 2009;234:1212–1219. doi: 10.3181/0812-RM-349. [PubMed] [CrossRef] [Google Scholar]23. Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta SP. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [PubMed] [CrossRef] [Google Scholar]24. Cai J, Kang Z, Liu WW, Luo X, Qiang S, Zhang JH, Ohta S, Sun X, Xu W, Tao H, Li R. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441:167–172. doi: 10.1016/j.neulet.2008.05.077. [PubMed] [CrossRef] [Google Scholar]25. Fu Y, Ito M, Fujita Y, Ito M, Ichihara M, Masuda A, Suzuki Y, Maesawa S, Kajita Y, Hirayama M, et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci Lett. 2009;453:81–85. doi: 10.1016/j.neulet.2009.02.016. [PubMed] [CrossRef] [Google Scholar]26. Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol. 2009;64:753–761. doi: 10.1007/s00280-008-0924-2. [PubMed] [CrossRef] [Google Scholar]27. Liu Y, Yang L, Tao K, Vizcaychipi MP, Lloyd DM, Sun X, Irwin MG, Ma D, Yu W. Protective effects of hydrogen enriched saline on liver ischemia reperfusion injury by reducing oxidative stress and HMGB1 release. BMC Gastroenterol. 2014;14:12. doi: 10.1186/1471-230X-14-12. [PMC free article] [PubMed] [CrossRef] [Google Scholar]28. Liu Q, Shen WF, Sun HY, Fan DF, Nakao A, Cai JM, Yan G, Zhou WP, Shen RX, Yang JM, Sun XJ. Hydrogen-rich saline protects against liver injury in rats with obstructive jaundice. Liver Int. 2010;30:958–968. doi: 10.1111/j.1478-3231.2010.02254.x. [PubMed] [CrossRef] [Google Scholar]29. Xu XF, Zhang J. Saturated hydrogen saline attenuates endotoxin-induced acute liver dysfunction in rats. Physiol Res. 2013;62:395–403. [PubMed] [Google Scholar]30. Levitt MD, Bond JH., Jr Volume, composition, and source of intestinal gas. Gastroenterology. 1970;59:921–929. [PubMed] [Google Scholar]Yang Q, Ji G, Pan R, et al. Protective effect of hydrogen-rich water on liver function of colorectal cancer patients treated with mFOLFOX6 chemotherapy. Mol Clin Oncol. 2017 Nov;7(5):891-896.