Hydrogen gas restores cells in patients with cancerScientific Research
Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis
https://doi.org/10.3892/or.2018.6841
Hideo Baba, Junji Akagi
Introduction
In cancer patients, treatment strategies, outcomes and prognosis are largely dependent on immune status and should therefore be monitored, ideally using peripheral blood markers that are easily measured non-invasively. The cytotoxic effector clusters of differentiated T cells are depleted by persistent stimulation by tumor antigens, resulting in a halt in proliferation, cytokine production, and immune activity. Programmed cell death 1 has been proposed as a marker for depleted T cells. PD-1 is abundantly expressed in circulating CD8+ T cells and tumor-infiltrating lymphocytes of cancer patients and is associated with poor prognosis in various cancers, including breast, pancreatic, and gastric cancers, characterized by persistent loss of mitochondrial function and mass, Peroxisome proliferator-activated receptor γ- is usually found in the progressive loss of coactivator 1α.
molecular hydrogen, a. H. Dihydrogen or H2 has been reported to effectively neutralize hydroxyl radicals (•OH) but not other reactive oxygen species, including superoxide anion (O2•-), hydrogen peroxide (H2O2), and nitric oxide (NO). •). (9). Consequently, hydrogen is now believed to reduce oxidative stress and ischemia-reperfusion injury in the brain, spinal cord (10), myocardium (11), intestinal epithelium (12), retina, testis (13), and kidney (14). Hydrogen has been used to treat a variety of diseases associated with oxidative stress, including trauma (15), neurodegenerative diseases (16), inflammatory diseases (17), organ transplantation, metabolic syndrome (18), diabetes (19), sepsis (20). . and burns (21), side effects of chemotherapy (22), radiation damage (23), hearing loss, and preeclampsia (24).
There are several studies on the preventive and therapeutic effects of hydrogen in various diseases including cancer (25-27). In particular, molecular hydrogen has been reported to activate PGC-1α (28), a positive regulator of mitochondrial biosynthesis and respiration, adaptive thermogenesis, gluconeogenesis, and many other metabolic processes (29), suggesting that it can CD8+ T cells rescued from mitochondrial dysfunction.
Therefore, this study examined whether PD-1 expression in circulating CD8+ T cells from 55 colorectal cancer patients was associated with progression-free survival (PFS) and overall survival (OS), and whether hydrogen PD-1 could affect prognostic improvement . + CD8+ T lymphocytes.
Materials and methods
Patients, sample collection and processing
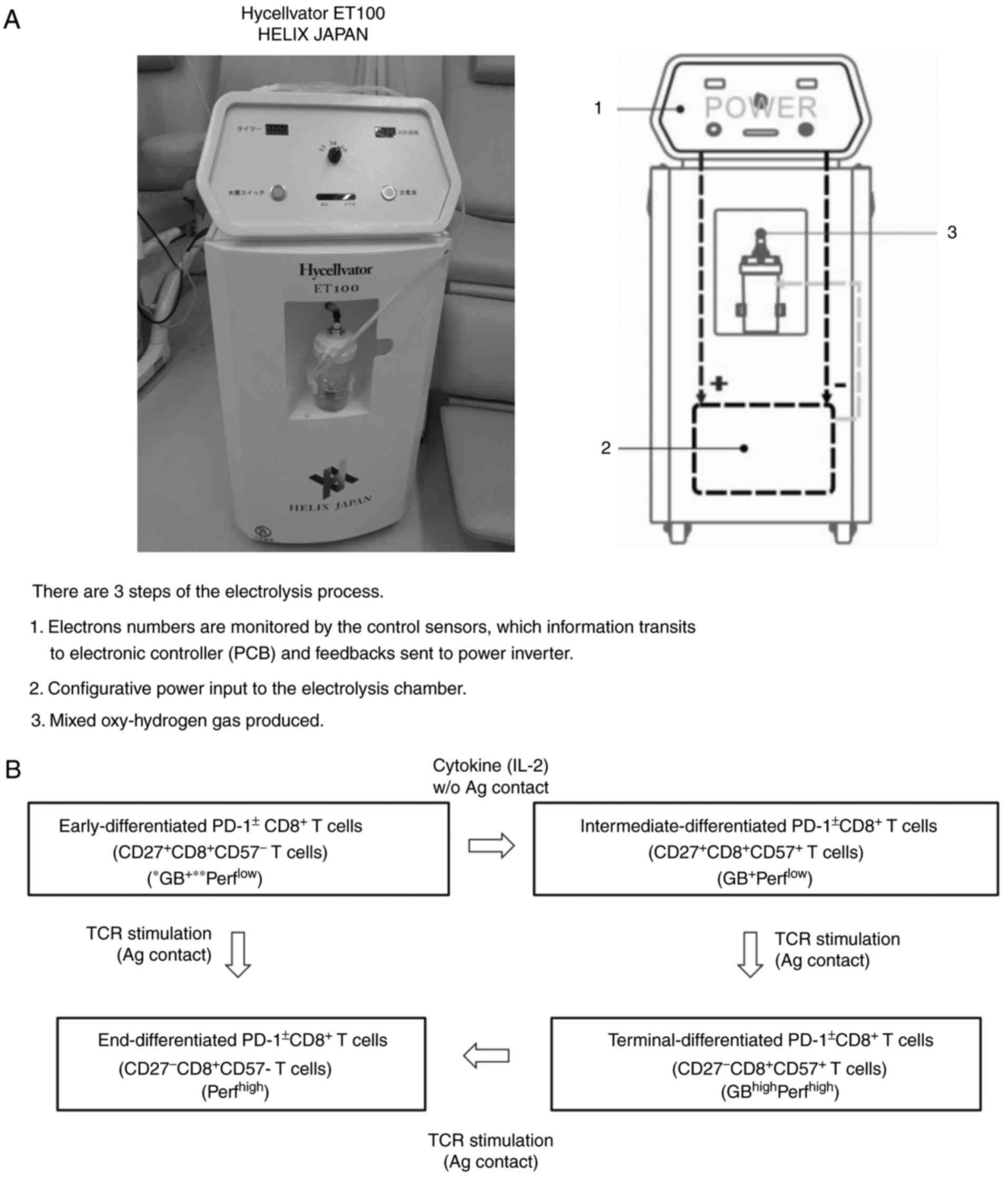 | Figure 1.(A) Hycellvator ET100 (Helix Japan, Co., Ltd.), which generates hydrogen gas. (B) Differentiation pathway of CD8+ T cells from early-differentiated to end-differentiated CD8+ T cells (via intermediate- and terminal-differentiated CD8+ T cells). PCB, printed circuit board; CD, cluster of differentiation; GB, granzyme; Perf, perforin. |
Table I.Comparison of clinicopathological data between patients with high- and low-terminal PD-1+ CD8+ T cells. |
Results
Circulating terminal PD-1+ CD8+ T cells are critical for prognosis in colorectal cancer
Under normal conditions, early CD8+ T cells express abundant PD-1, which gradually decreases with differentiation into terminal CD8+ T cells. However, loss of PD-1 is delayed in cancer patients and may lead to poor prognosis. Therefore, Cox proportional hazards regression analysis was used to estimate PD-1+/- CD8+ T cell subsets (Figure 1B) and clinicopathological factors [age, sex, primary tumor (T), regional lymph nodes (N)] to identify distant Metastasis (M) and histology] were associated with PFS and OS in patients with stage IV colorectal cancer. Univariate analysis of 18 factors, including 6 clinicopathological factors, found that terminal PD-1+ CD8+ T cells were significantly associated with worse PFS [hazard ratio (HR), 1.239; Under normal conditions, early CD8+ T cells express abundant PD-1, which gradually declines as they differentiate into terminal CD8+ T cells. However, loss of PD-1 in cancer patients is delayed and may lead to a poor prognosis. Therefore, Cox proportional hazards regression analysis was used to determine PD-1+/- CD8+ T cell subsets (Figure 1B) and clinicopathological factors [age, sex, primary tumor (T), regional lymph nodes (N)]. metastasis (M) and histology] were associated with PFS and OS in patients with stage IV colorectal cancer. Univariate analysis of 18 factors, including 6 clinicopathological factors, revealed that terminal PD-1+ CD8+ T cells were significantly associated with worse PFS [hazard ratio (HR), 1.239; 95% confidence interval (CI), 1.106-1.389; P<0.0001] and OS (HR, 1.183; 95% CI, 1.066-1.314; P=0.002) and terminal PD-1+ CD8+ T cells. (PFS: HR, 1.296; 95% CI, 1.053-1.595 p=0.015 OS: HR 1.333 95% CI 1.103-1.610 p=0.003). In contrast, early PD-1 and late PD-1 CD8+ T cells were better (HR, 0.961; 95% CI, 0.926-0.997; p=0.036) and worse (HR, 1.044; 95) compared to – % CI, 1.005) 1.084; P=0.025) operating system. Univariate analysis data for the other 14 factors of PFS and OS are as follows: age, P=0.270 and P=0.886; sex, P=0.894 and P=0.398; T factor, P=0.332 and P=0.664; N factor, P =0.080 and P=0.150; distant metastases, univariate analysis not possible); histology, P=0.503 and P=0.184; early CD8+ T cells, P=0.953 and P=0.273; early PD-1+ CD8+ T cells , P=0.757 and P=0.560; intermediate CD8+ T cells, P=0.434 and P=0.560; intermediate PD-1+ CD8+ T cells, P=0.799 and P=0.505; intermediate PD-1 CD8+ T cells, P=0.137 and P=0.099; terminal CD8+ T cells, P=0.453 and P=0.595; terminal PD-1 CD8+ T cells, P=0.681 and P=0.886; and terminal CD8+ T cells, P=0.285 and P=0.566.
Based on multivariate Cox regression, terminal PD-1+ CD8+ T cells were more effectively associated with PFS (HR, 1.239; 95% CI, 1.106-1.389; p<0.0001) and OS (HR, 1.136; 95% CI, 1.019-) . 1.266; P = 0.022) than the others. Multivariate data for the other 3 factors are as follows: early PD-1 CD8+ T cells, PFS P=0.677, OS P=0.352; late PD-1+ CD8+ T cells, for PFS, P=0.274; HR, 1.247; 95 % CI, 1.007-1.543; p=0.043; and terminal PD-1 CD8+ T cells, PFS was P=0.561, OS was P=0.206). Thus, the cutoff values for PFS and OS determined by the defined receptor operating curves were 8.18% and 6.81%, respectively, and patients were stratified by frequency of terminal PD-1+ CD8+ T cells (Figure 1). 2A and B). Clinicopathological factors did not differ significantly between patients with high and low terminal PD-1+ CD8+ T cells (Table I).The resulting stratified Kaplan-Meier survival curves showed that patients with terminal PD-1+ CD8+ T cells had higher PFS (log-rank test, P=0.001) and OS (log-rank test, P=0.008) than off (log-rank test, P=0.008). Rank test, P=0.008). Figure 2C and D). Patients with a high proportion of terminal PD-1+ CD8+ T cells had a median time to PFS of 18 months, compared with the proportion of patients with a low proportion of terminal PD-1+ CD8+ T cells (>50% of patients survived), In contrast, patients with a high proportion of terminal PD-1+ CD8+ T cells had an OS of 18 months, while those with a low proportion of terminal PD-1+ CD8+ T cells had an OS of 46 months.
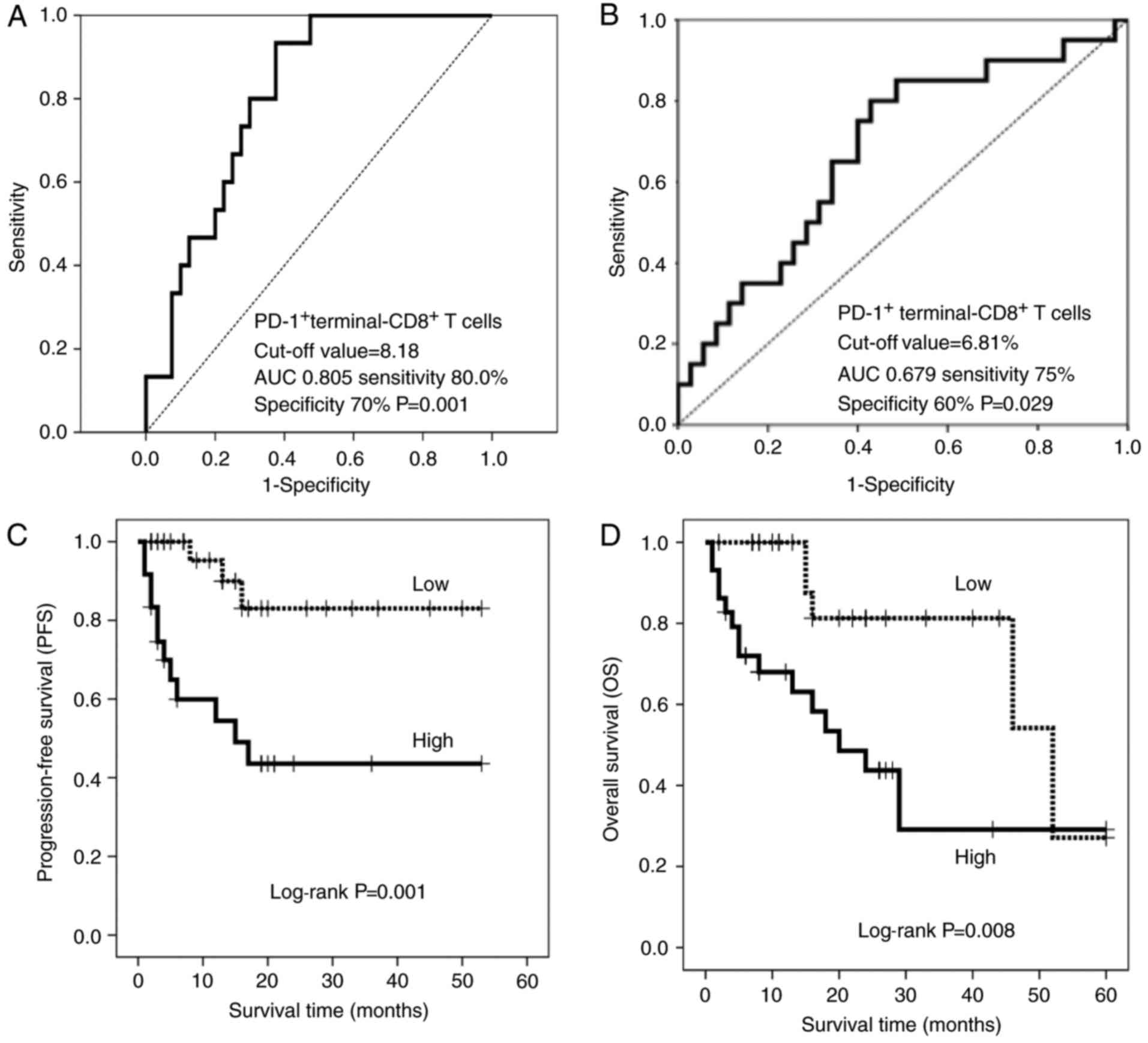 | Figure 2.Receiver operating characteristic curves of (A) PFS and (B) OS based on terminal PD-1+ CD8+ T cells. The cut-off values are 8.18 and 6.81%, respectively. (C) PFS and (D) OS in patients with a high- (solid line) and low- (dotted line) percentage of terminal PD-1+ CD8+ T cells. PFS, progression-free survival; OS, overall survival; AUC, area under the curve; PD-1, programmed cell death 1; CD, cluster of differentiation. |
Hydrogen gas reduces the proportion of PD-1+ CD8+ T cells and improves prognosis
Molecular hydrogen has been reported to activate PGC-1α (28) and increase mitochondrial activity (29), thereby rescuing depleted CD8+ T cells with inactivated mitochondria following progressive loss of PGC-1α (15). Therefore, this study examined whether hydrogen gas alters the ratio of PD-1+/- CD8+ T cell subsets and whether this alteration, if any, is associated with prognosis in patients with stage IV cancer. Specifically, hydrogen reduced early, intermediate, terminal, and terminal PD-1+ levels by 27 (49.1%), 28 (50.9%), 35 (63.6%), and 32 (58.2%) levels of CD8+, T cells in 55 patients. In contrast, hydrogen increased early, intermediate, end and end PD- 1 CD8+ T.The ratio of intermediate PD-1+ CD8+ T cells to pretreatment (proportion of intermediate PD-1+ CD8+ T cells) after hydrogen treatment was significantly associated with PFS (HR, 2.286; 95% CI, 1.284-4.070; p=0.005) and OS (HR, 2.398; 95% CI, 1.384-4.156; p=0.002). Similar correlations were observed for the mean ratio of PD-1 CD8+ T cells (PFS: HR, 2.286; 95% CI, 1.284-4.070; p=0.005; OS: HR, 2.398; 95% CI, 1.384-4.156; p = 0.002) and terminal PD-1+ CD8+ T cell ratio (PFS: HR, 6.459; 95% CI, 2.384-17.50; p < 0.0001; OS: HR, 4.158; 95% CI, 1.718-10.06; p= 0.002) . ) . In contrast, the proportion of early PD-1 CD8+ T cells predicted worse OS time (HR, 2.398; 95% CI, 1.384-4.156; p=0.002), while terminal PD-1 CD8+ T cells The proportion of cells was associated with longer overall survival (HR, 0.002; 95% CI, 0.000-0.131; p=0.004). Multivariate analysis found that the proportion of terminal PD-1+ CD8+ T cells was an independent predictor of poor PFS (HR, 6.459; 95% CI, 2.384-17.50; p<0.0001) and OS (HR, 2.957, 95) . %CI, 1.185-7.378; p = 0.020). Based on these results, patients with high and low ratios of PD-1+ CD8+ terminal T cells were stratified using cutoffs of 0.87% and 0.77% for PFS and OS, respectively (Figure 3A and B). Clinicopathological factors did not differ significantly between patients with high and low terminal PD-1+ CD8+ T cell ratios (Table II). The resulting stratified PFS and OS curves are shown in Figures 3C and D, indicating that patients with low terminal PD-1+ CD8+ T cell ratios had significantly longer PFS (P<0.0001) and OS time (P=0.004) Have the opportunity. Patients with high terminal PD-1+ CD8+ T cell ratios had a median follow-up of 13 months, compared with 13 months for patients with low terminal PD-1+ (>50% of patients survived). Patients with a high proportion of terminal PD-1+ CD8+ T cells had an OS of 15 months, while those with a low proportion of terminal PD-1+ CD8+ T cells had an OS of 46 months.
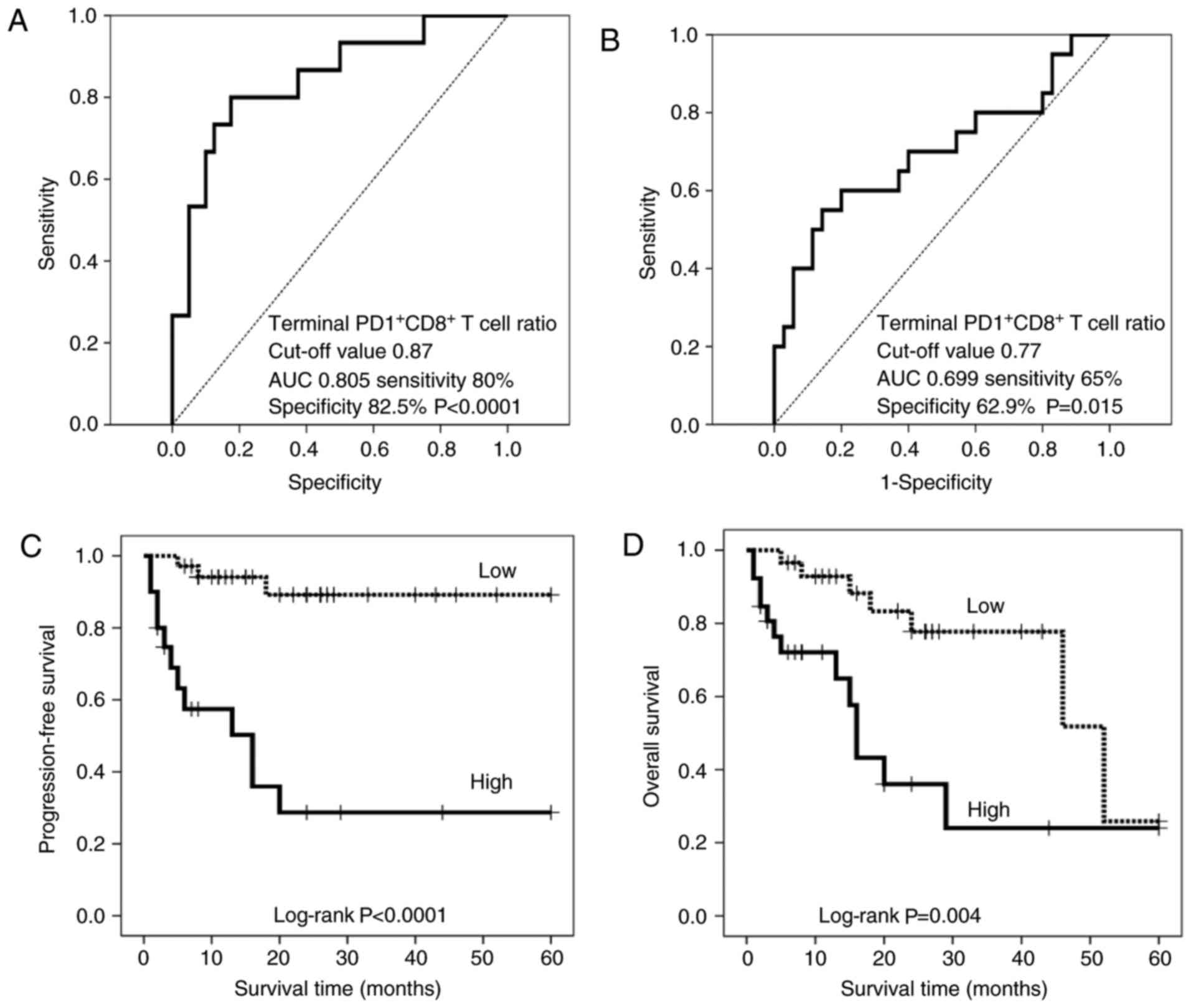 | Figure 3.Analysis of the ratio of terminal PD-1+/− CD8+ T cells prior to and following treatment with hydrogen gas (terminal PD-1+/− CD8+ T cell ratio). Receiver operating characteristic curve of (A) PFS and (B) OS based on the terminal PD-1+ CD8+ T cell ratio. The cut-off values are 0.87 and 0.77%, respectively. (C) PFS and (D) OS in patients with a high-(solid line) and low-(dotted line) terminal PD-1+ CD8+ T cell ratio. PFS, progression-free survival; OS, overall survival; AUC, area under the curve; PD-1, programmed cell death 1; CD, cluster of differentiation. |
Table II.Comparison of clinicopathological data between patients with high- and low-terminal PD-1+ CD8+ T cell ratio (the ratio of terminal PD-1+ CD8+ T cells following the hydrogen treatment to prior to it). |
Hydrogen-induced accumulation of terminal PD-1− CD8+ T cells is associated with an improved prognosis
Based on the hypothesis that hydrogen can activate mitochondrial function and convert depleted PD-1+ CD8+ terminal T cells into active PD-1 CD8+ terminal T cells, this study explored whether altering the frequency of hydrogen could affect the latter in patients receiving hydrogen Prognosis. Therefore, patients were stratified according to the terminal PD-1 CD8+ T cell ratio according to cutoff values of 1.01 and 1.02 for PFS (Fig. 4A) and OS (Fig. 4B), respectively. Clinicopathological factors did not differ significantly between patients with high and low terminal PD-1 CD8+ T cell ratios (Table III). The resulting stratified survival curves are shown in Figure 4C and D, respectively, indicating that the high-ratio patients had significantly longer PFS (P=0.004) and OS (P=0.024) times compared with the low-ratio patients. Among patients with lower terminal PD-1 CD8+ T cell ratios, median follow-up was 15 months.However, patients with a high terminal PD-1 CD8+ T cell ratio were not achieved (>50% of patients survived), whereas patients with a low terminal PD-1 CD8+ T cell ratio had an OS of 16 months and 52, with a high percentage of Patients with PD-1-CD8+ T cells have to spend several months. Furthermore, hydrogen therapy significantly prolonged PFS time in patients with low scores (P=0.014) and generally longer but not significant OS time compared with patients with high terminal PD-1 CD8+ T cell scores (P=0.014). 0.165), although there was no significant difference between the groups before treatment (Figure 5A and C).
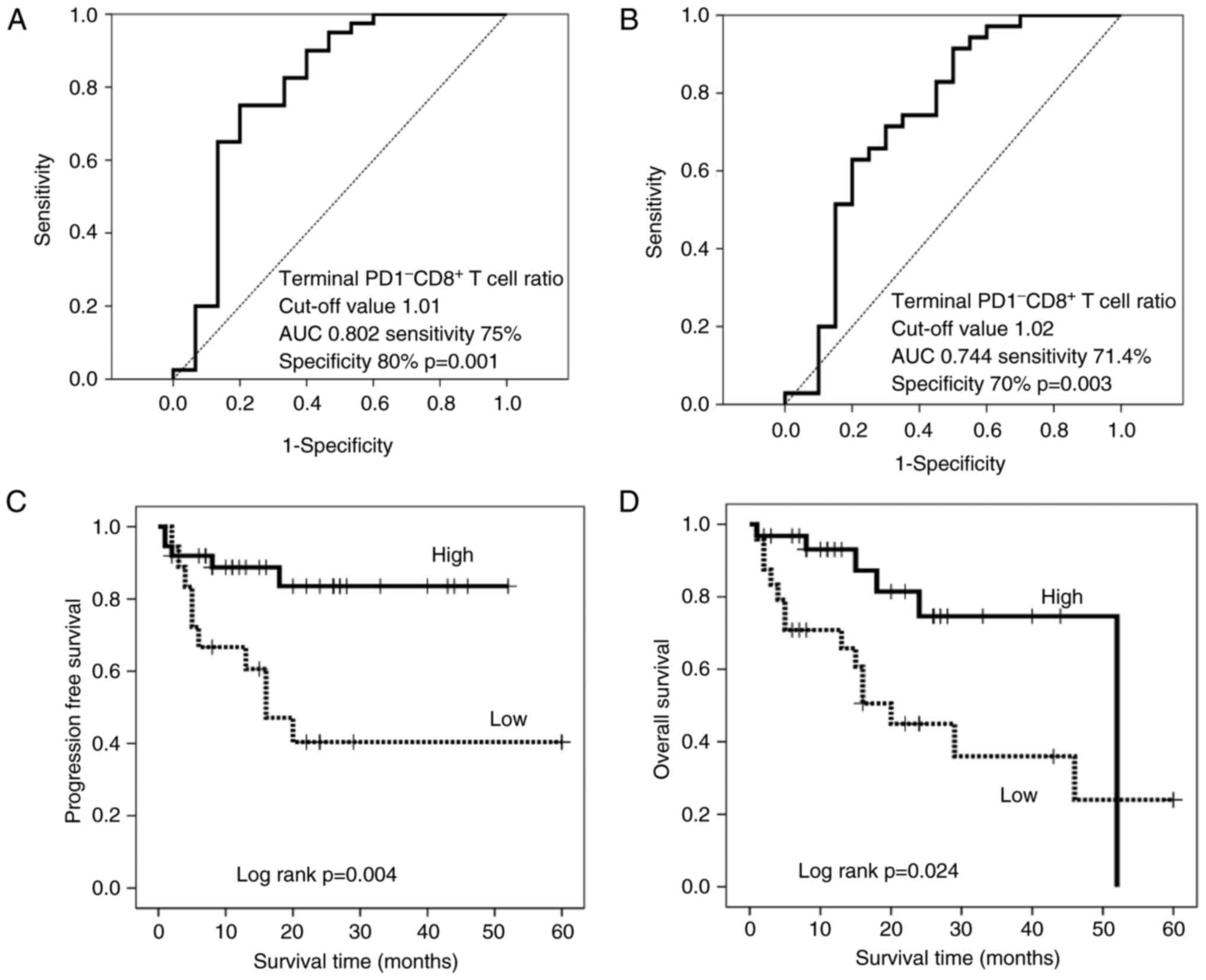 | Figure 4.Receiver operating characteristic curve of (A) PFS and (B) OS based on the terminal PD-1− CD8+ T cell ratio, with cut-off values of 1.01 and 1.02%, respectively. (C) PFS and (D) OS in patients with a high- (solid line) and low- (dotted line) terminal PD-1− CD8+ T cell ratio. PFS, progression-free survival; OS, overall survival; AUC, area under the curve; PD-1, programmed cell death 1; CD, cluster of differentiation. |
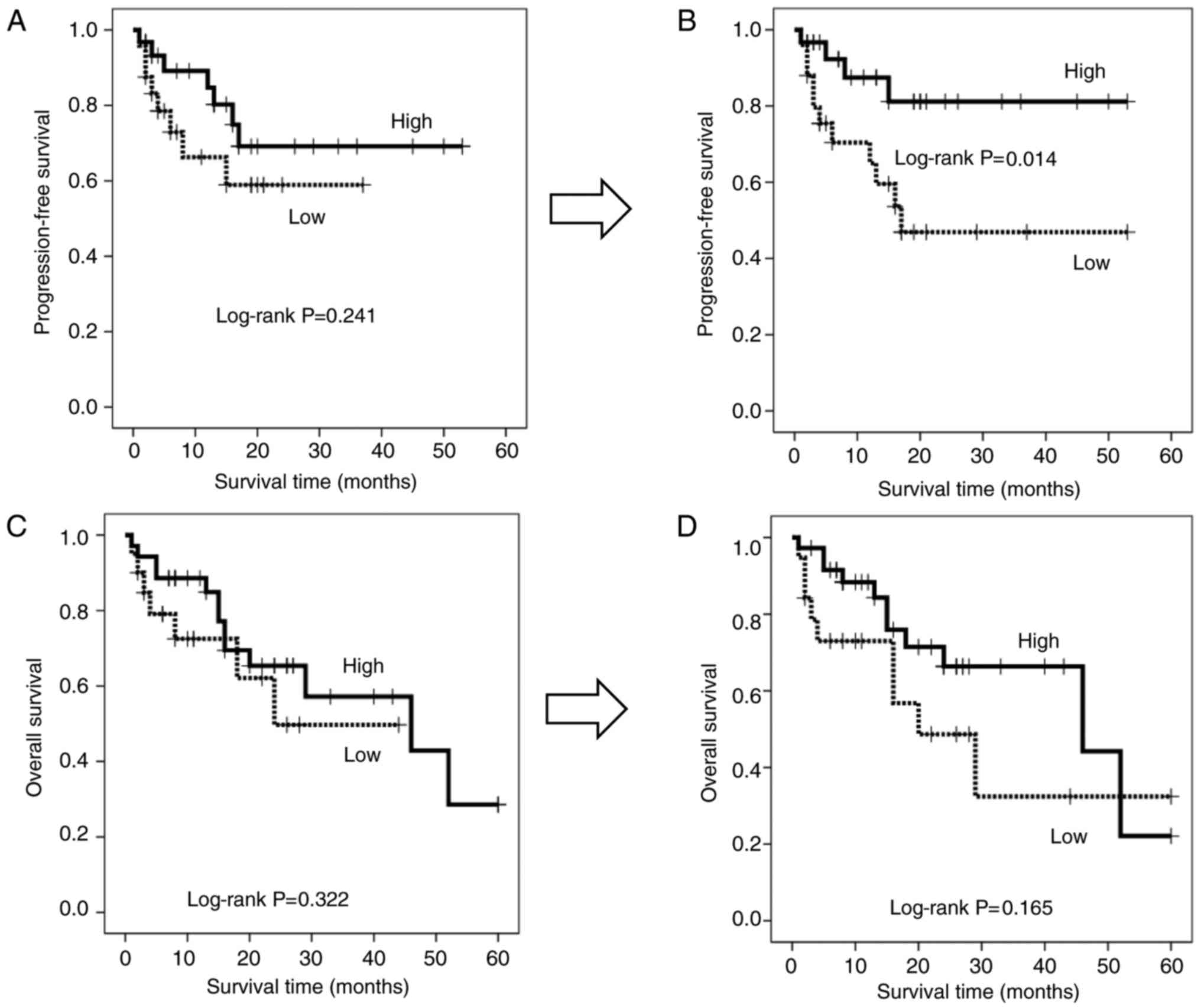 | Figure 5.Progression-free survival in patients with a high-(solid line) and low-(dotted line) terminal PD-1− CD8+ T cell ratio (A) prior to and (B) following treatment with hydrogen gas. Comparison of overall survival between patients with a high-(solid line) and low-(dotted line) terminal PD-1− CD8+ T cell ratio (C) prior to and (D) following treatment with hydrogen gas. PD-1, programmed cell death 1; CD, cluster of differentiation. |
Table III.Comparison of clinicopathological data between patients with high- and low-terminal PD-1− CD8+ T cell ratio. |
Serum terminal PD-1+/− CD8+ T cells are important immune indices in patients with advanced cancer
As previously described, hydrogen decreased the proportion of PD1+CD8+ terminal T cells, but increased the frequency of PD1-CD8+ terminal T cells, and the magnitude of these changes was strongly associated with prognosis. In particular, PD1+-CD8+ terminal T cells were significantly negatively correlated with PD1-CD8+ terminal T cells (Figure 6A and B), suggesting that the dynamic balance between these subsets has a significant impact on the prognosis of patients with advanced colorectal cancer. great contribution. Therefore, patients were stratified according to all possible high/low combinations of these subgroups [categories (Cat.) 1-4; first category:Patients with low PD1+ and high PD1 terminal CD8+ T cells; Category 2: High PD1+ and high PD1- terminal CD8+ T cells; Cat. 3: Patients with low PD1+ and low PD1 terminal CD8+ T cells; ; Cat. 4: Patient with high PD1+ and high PD1- terminal CD8+ T cells; Figure 6A and B]. Kaplan-Meier analysis showed that category 1 patients had significantly longer PFS times than all other groups, while category 3 patients had significantly longer OS times. In contrast, the PFS (Fig. 6C) and OS (Fig. 6D) times were significantly lower in category 4 patients than in other patients. Hydrogen also increased the number of patients with low PD1+-terminal CD8+ T cells (types 1 and 3), but decreased the number of patients with high PD1+-terminal CD8+ T cells (types 2 and 4), thereby reducing stage IV The prognosis of colorectal cancer patients improved (Figure 6A and B; Table IV).
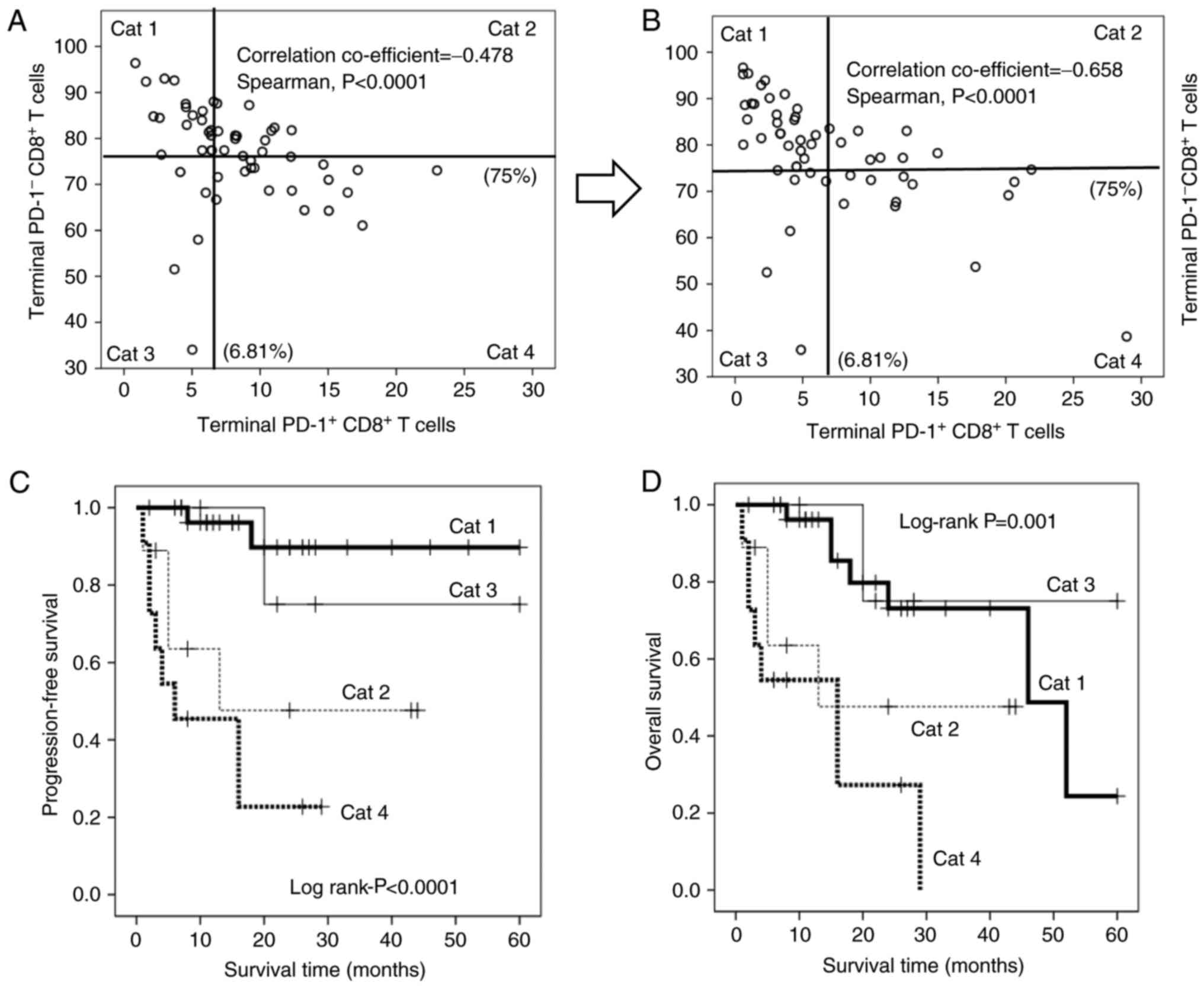 | Figure 6.Point diagram of the correlation between terminal PD-1+ (x-axis) and PD-1− CD8+ T cells (y-axis) (A) prior to and (B) following treatment with hydrogen gas. The x-axis of terminal PD-1+ and the y-axis of PD-1− CD8+ T cells are divided into two sections with cut-offs of 6.81 and 75%, respectively. Cat 1: Low-terminal PD-1+ CD8+ T cells and high-terminal PD-1− CD8+ T cells; Cat 2: High-terminal PD-1+ CD8+ T cells and high-terminal PD-1− CD8+ T cells; Cat 3: Low-terminal PD-1+ CD8+ T cells and low-terminal PD-1− CD8+ T cells; Cat 4: High-terminal PD-1+ CD8+ T cells and low-terminal PD-1 CD8+ T cells. (C) Progression-free survival and (D) overall survival in Cat 1 (bold line), Cat 2 (thin dotted line), Cat 3 (thin line) and Cat 4 (bold dotted line) patients. PD-1, programmed cell death 1; CD, cluster of differentiation; Cat, category. |
Table IV.Change of category following hydrogen gas treatment. |
Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis
Authors: Hideo Baba, Junji Akagi
View Affiliations
Published online on: November 1, 2018
DOI: 10.3892
Published on: 20180111
Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis
https://doi.org/10.3892/or.2018.6841
Hideo Baba, Junji Akagi
Introduction
In cancer patients, treatment strategies, outcomes and prognosis are largely dependent on immune status and should therefore be monitored, ideally using peripheral blood markers that are easily measured non-invasively. The cytotoxic effector clusters of differentiated T cells are depleted by persistent stimulation by tumor antigens, resulting in a halt in proliferation, cytokine production, and immune activity. Programmed cell death 1 has been proposed as a marker for depleted T cells. PD-1 is abundantly expressed in circulating CD8+ T cells and tumor-infiltrating lymphocytes of cancer patients and is associated with poor prognosis in various cancers, including breast, pancreatic, and gastric cancers, characterized by persistent loss of mitochondrial function and mass, Peroxisome proliferator-activated receptor γ- is usually found in the progressive loss of coactivator 1α.
molecular hydrogen, a. H. Dihydrogen or H2 has been reported to effectively neutralize hydroxyl radicals (•OH) but not other reactive oxygen species, including superoxide anion (O2•-), hydrogen peroxide (H2O2), and nitric oxide (NO). •). (9). Consequently, hydrogen is now believed to reduce oxidative stress and ischemia-reperfusion injury in the brain, spinal cord (10), myocardium (11), intestinal epithelium (12), retina, testis (13), and kidney (14). Hydrogen has been used to treat a variety of diseases associated with oxidative stress, including trauma (15), neurodegenerative diseases (16), inflammatory diseases (17), organ transplantation, metabolic syndrome (18), diabetes (19), sepsis (20). . and burns (21), side effects of chemotherapy (22), radiation damage (23), hearing loss, and preeclampsia (24).
There are several studies on the preventive and therapeutic effects of hydrogen in various diseases including cancer (25-27). In particular, molecular hydrogen has been reported to activate PGC-1α (28), a positive regulator of mitochondrial biosynthesis and respiration, adaptive thermogenesis, gluconeogenesis, and many other metabolic processes (29), suggesting that it can CD8+ T cells rescued from mitochondrial dysfunction.
Therefore, this study examined whether PD-1 expression in circulating CD8+ T cells from 55 colorectal cancer patients was associated with progression-free survival (PFS) and overall survival (OS), and whether hydrogen PD-1 could affect prognostic improvement . + CD8+ T lymphocytes.
Materials and methods
Patients, sample collection and processing
Figure 1.
(A) Hycellvator ET100 (Helix Japan, Co., Ltd.), which generates hydrogen gas. (B) Differentiation pathway of CD8+ T cells from early-differentiated to end-differentiated CD8+ T cells (via intermediate- and terminal-differentiated CD8+ T cells). PCB, printed circuit board; CD, cluster of differentiation; GB, granzyme; Perf, perforin.
Table I.
Comparison of clinicopathological data between patients with high- and low-terminal PD-1+ CD8+ T cells.
Results
Circulating terminal PD-1+ CD8+ T cells are critical for prognosis in colorectal cancer
Under normal conditions, early CD8+ T cells express abundant PD-1, which gradually decreases with differentiation into terminal CD8+ T cells. However, loss of PD-1 is delayed in cancer patients and may lead to poor prognosis. Therefore, Cox proportional hazards regression analysis was used to estimate PD-1+/- CD8+ T cell subsets (Figure 1B) and clinicopathological factors [age, sex, primary tumor (T), regional lymph nodes (N)] to identify distant Metastasis (M) and histology] were associated with PFS and OS in patients with stage IV colorectal cancer. Univariate analysis of 18 factors, including 6 clinicopathological factors, found that terminal PD-1+ CD8+ T cells were significantly associated with worse PFS [hazard ratio (HR), 1.239; Under normal conditions, early CD8+ T cells express abundant PD-1, which gradually declines as they differentiate into terminal CD8+ T cells. However, loss of PD-1 in cancer patients is delayed and may lead to a poor prognosis. Therefore, Cox proportional hazards regression analysis was used to determine PD-1+/- CD8+ T cell subsets (Figure 1B) and clinicopathological factors [age, sex, primary tumor (T), regional lymph nodes (N)]. metastasis (M) and histology] were associated with PFS and OS in patients with stage IV colorectal cancer. Univariate analysis of 18 factors, including 6 clinicopathological factors, revealed that terminal PD-1+ CD8+ T cells were significantly associated with worse PFS [hazard ratio (HR), 1.239; 95% confidence interval (CI), 1.106-1.389; P<0.0001] and OS (HR, 1.183; 95% CI, 1.066-1.314; P=0.002) and terminal PD-1+ CD8+ T cells. (PFS: HR, 1.296; 95% CI, 1.053-1.595 p=0.015 OS: HR 1.333 95% CI 1.103-1.610 p=0.003). In contrast, early PD-1 and late PD-1 CD8+ T cells were better (HR, 0.961; 95% CI, 0.926-0.997; p=0.036) and worse (HR, 1.044; 95) compared to – % CI, 1.005) 1.084; P=0.025) operating system. Univariate analysis data for the other 14 factors of PFS and OS are as follows: age, P=0.270 and P=0.886; sex, P=0.894 and P=0.398; T factor, P=0.332 and P=0.664; N factor, P =0.080 and P=0.150; distant metastases, univariate analysis not possible); histology, P=0.503 and P=0.184; early CD8+ T cells, P=0.953 and P=0.273; early PD-1+ CD8+ T cells , P=0.757 and P=0.560; intermediate CD8+ T cells, P=0.434 and P=0.560; intermediate PD-1+ CD8+ T cells, P=0.799 and P=0.505; intermediate PD-1 CD8+ T cells, P=0.137 and P=0.099; terminal CD8+ T cells, P=0.453 and P=0.595; terminal PD-1 CD8+ T cells, P=0.681 and P=0.886; and terminal CD8+ T cells, P=0.285 and P=0.566.
Based on multivariate Cox regression, terminal PD-1+ CD8+ T cells were more effectively associated with PFS (HR, 1.239; 95% CI, 1.106-1.389; p<0.0001) and OS (HR, 1.136; 95% CI, 1.019-) . 1.266; P = 0.022) than the others. Multivariate data for the other 3 factors are as follows: early PD-1 CD8+ T cells, PFS P=0.677, OS P=0.352; late PD-1+ CD8+ T cells, for PFS, P=0.274; HR, 1.247; 95 % CI, 1.007-1.543; p=0.043; and terminal PD-1 CD8+ T cells, PFS was P=0.561, OS was P=0.206). Thus, the cutoff values for PFS and OS determined by the defined receptor operating curves were 8.18% and 6.81%, respectively, and patients were stratified by frequency of terminal PD-1+ CD8+ T cells (Figure 1). 2A and B). Clinicopathological factors did not differ significantly between patients with high and low terminal PD-1+ CD8+ T cells (Table I).The resulting stratified Kaplan-Meier survival curves showed that patients with terminal PD-1+ CD8+ T cells had higher PFS (log-rank test, P=0.001) and OS (log-rank test, P=0.008) than off (log-rank test, P=0.008). Rank test, P=0.008). Figure 2C and D). Patients with a high proportion of terminal PD-1+ CD8+ T cells had a median time to PFS of 18 months, compared with the proportion of patients with a low proportion of terminal PD-1+ CD8+ T cells (>50% of patients survived), In contrast, patients with a high proportion of terminal PD-1+ CD8+ T cells had an OS of 18 months, while those with a low proportion of terminal PD-1+ CD8+ T cells had an OS of 46 months.
Figure 2.
Receiver operating characteristic curves of (A) PFS and (B) OS based on terminal PD-1+ CD8+ T cells. The cut-off values are 8.18 and 6.81%, respectively. (C) PFS and (D) OS in patients with a high- (solid line) and low- (dotted line) percentage of terminal PD-1+ CD8+ T cells. PFS, progression-free survival; OS, overall survival; AUC, area under the curve; PD-1, programmed cell death 1; CD, cluster of differentiation.
Hydrogen gas reduces the proportion of PD-1+ CD8+ T cells and improves prognosis
Molecular hydrogen has been reported to activate PGC-1α (28) and increase mitochondrial activity (29), thereby rescuing depleted CD8+ T cells with inactivated mitochondria following progressive loss of PGC-1α (15). Therefore, this study examined whether hydrogen gas alters the ratio of PD-1+/- CD8+ T cell subsets and whether this alteration, if any, is associated with prognosis in patients with stage IV cancer. Specifically, hydrogen reduced early, intermediate, terminal, and terminal PD-1+ levels by 27 (49.1%), 28 (50.9%), 35 (63.6%), and 32 (58.2%) levels of CD8+, T cells in 55 patients. In contrast, hydrogen increased early, intermediate, end and end PD- 1 CD8+ T.The ratio of intermediate PD-1+ CD8+ T cells to pretreatment (proportion of intermediate PD-1+ CD8+ T cells) after hydrogen treatment was significantly associated with PFS (HR, 2.286; 95% CI, 1.284-4.070; p=0.005) and OS (HR, 2.398; 95% CI, 1.384-4.156; p=0.002). Similar correlations were observed for the mean ratio of PD-1 CD8+ T cells (PFS: HR, 2.286; 95% CI, 1.284-4.070; p=0.005; OS: HR, 2.398; 95% CI, 1.384-4.156; p = 0.002) and terminal PD-1+ CD8+ T cell ratio (PFS: HR, 6.459; 95% CI, 2.384-17.50; p < 0.0001; OS: HR, 4.158; 95% CI, 1.718-10.06; p= 0.002) . ) . In contrast, the proportion of early PD-1 CD8+ T cells predicted worse OS time (HR, 2.398; 95% CI, 1.384-4.156; p=0.002), while terminal PD-1 CD8+ T cells The proportion of cells was associated with longer overall survival (HR, 0.002; 95% CI, 0.000-0.131; p=0.004). Multivariate analysis found that the proportion of terminal PD-1+ CD8+ T cells was an independent predictor of poor PFS (HR, 6.459; 95% CI, 2.384-17.50; p<0.0001) and OS (HR, 2.957, 95) . %CI, 1.185-7.378; p = 0.020). Based on these results, patients with high and low ratios of PD-1+ CD8+ terminal T cells were stratified using cutoffs of 0.87% and 0.77% for PFS and OS, respectively (Figure 3A and B). Clinicopathological factors did not differ significantly between patients with high and low terminal PD-1+ CD8+ T cell ratios (Table II). The resulting stratified PFS and OS curves are shown in Figures 3C and D, indicating that patients with low terminal PD-1+ CD8+ T cell ratios had significantly longer PFS (P<0.0001) and OS time (P=0.004) Have the opportunity. Patients with high terminal PD-1+ CD8+ T cell ratios had a median follow-up of 13 months, compared with 13 months for patients with low terminal PD-1+ (>50% of patients survived). Patients with a high proportion of terminal PD-1+ CD8+ T cells had an OS of 15 months, while those with a low proportion of terminal PD-1+ CD8+ T cells had an OS of 46 months.
Figure 3.
Analysis of the ratio of terminal PD-1+/− CD8+ T cells prior to and following treatment with hydrogen gas (terminal PD-1+/− CD8+ T cell ratio). Receiver operating characteristic curve of (A) PFS and (B) OS based on the terminal PD-1+ CD8+ T cell ratio. The cut-off values are 0.87 and 0.77%, respectively. (C) PFS and (D) OS in patients with a high-(solid line) and low-(dotted line) terminal PD-1+ CD8+ T cell ratio. PFS, progression-free survival; OS, overall survival; AUC, area under the curve; PD-1, programmed cell death 1; CD, cluster of differentiation.
Table II.
Comparison of clinicopathological data between patients with high- and low-terminal PD-1+ CD8+ T cell ratio (the ratio of terminal PD-1+ CD8+ T cells following the hydrogen treatment to prior to it).
Hydrogen-induced accumulation of terminal PD-1− CD8+ T cells is associated with an improved prognosis
Based on the hypothesis that hydrogen can activate mitochondrial function and convert depleted PD-1+ CD8+ terminal T cells into active PD-1 CD8+ terminal T cells, this study explored whether altering the frequency of hydrogen could affect the latter in patients receiving hydrogen Prognosis. Therefore, patients were stratified according to the terminal PD-1 CD8+ T cell ratio according to cutoff values of 1.01 and 1.02 for PFS (Fig. 4A) and OS (Fig. 4B), respectively. Clinicopathological factors did not differ significantly between patients with high and low terminal PD-1 CD8+ T cell ratios (Table III). The resulting stratified survival curves are shown in Figure 4C and D, respectively, indicating that the high-ratio patients had significantly longer PFS (P=0.004) and OS (P=0.024) times compared with the low-ratio patients. Among patients with lower terminal PD-1 CD8+ T cell ratios, median follow-up was 15 months.However, patients with a high terminal PD-1 CD8+ T cell ratio were not achieved (>50% of patients survived), whereas patients with a low terminal PD-1 CD8+ T cell ratio had an OS of 16 months and 52, with a high percentage of Patients with PD-1-CD8+ T cells have to spend several months. Furthermore, hydrogen therapy significantly prolonged PFS time in patients with low scores (P=0.014) and generally longer but not significant OS time compared with patients with high terminal PD-1 CD8+ T cell scores (P=0.014). 0.165), although there was no significant difference between the groups before treatment (Figure 5A and C).
Figure 4.
Receiver operating characteristic curve of (A) PFS and (B) OS based on the terminal PD-1− CD8+ T cell ratio, with cut-off values of 1.01 and 1.02%, respectively. (C) PFS and (D) OS in patients with a high- (solid line) and low- (dotted line) terminal PD-1− CD8+ T cell ratio. PFS, progression-free survival; OS, overall survival; AUC, area under the curve; PD-1, programmed cell death 1; CD, cluster of differentiation.
Figure 5.
Progression-free survival in patients with a high-(solid line) and low-(dotted line) terminal PD-1− CD8+ T cell ratio (A) prior to and (B) following treatment with hydrogen gas. Comparison of overall survival between patients with a high-(solid line) and low-(dotted line) terminal PD-1− CD8+ T cell ratio (C) prior to and (D) following treatment with hydrogen gas. PD-1, programmed cell death 1; CD, cluster of differentiation.
Table III.
Comparison of clinicopathological data between patients with high- and low-terminal PD-1− CD8+ T cell ratio.
Serum terminal PD-1+/− CD8+ T cells are important immune indices in patients with advanced cancer
As previously described, hydrogen decreased the proportion of PD1+CD8+ terminal T cells, but increased the frequency of PD1-CD8+ terminal T cells, and the magnitude of these changes was strongly associated with prognosis. In particular, PD1+-CD8+ terminal T cells were significantly negatively correlated with PD1-CD8+ terminal T cells (Figure 6A and B), suggesting that the dynamic balance between these subsets has a significant impact on the prognosis of patients with advanced colorectal cancer. great contribution. Therefore, patients were stratified according to all possible high/low combinations of these subgroups [categories (Cat.) 1-4; first category:Patients with low PD1+ and high PD1 terminal CD8+ T cells; Category 2: High PD1+ and high PD1- terminal CD8+ T cells; Cat. 3: Patients with low PD1+ and low PD1 terminal CD8+ T cells; ; Cat. 4: Patient with high PD1+ and high PD1- terminal CD8+ T cells; Figure 6A and B]. Kaplan-Meier analysis showed that category 1 patients had significantly longer PFS times than all other groups, while category 3 patients had significantly longer OS times. In contrast, the PFS (Fig. 6C) and OS (Fig. 6D) times were significantly lower in category 4 patients than in other patients. Hydrogen also increased the number of patients with low PD1+-terminal CD8+ T cells (types 1 and 3), but decreased the number of patients with high PD1+-terminal CD8+ T cells (types 2 and 4), thereby reducing stage IV The prognosis of colorectal cancer patients improved (Figure 6A and B; Table IV).
Figure 6.
Point diagram of the correlation between terminal PD-1+ (x-axis) and PD-1− CD8+ T cells (y-axis) (A) prior to and (B) following treatment with hydrogen gas. The x-axis of terminal PD-1+ and the y-axis of PD-1− CD8+ T cells are divided into two sections with cut-offs of 6.81 and 75%, respectively. Cat 1: Low-terminal PD-1+ CD8+ T cells and high-terminal PD-1− CD8+ T cells; Cat 2: High-terminal PD-1+ CD8+ T cells and high-terminal PD-1− CD8+ T cells; Cat 3: Low-terminal PD-1+ CD8+ T cells and low-terminal PD-1− CD8+ T cells; Cat 4: High-terminal PD-1+ CD8+ T cells and low-terminal PD-1 CD8+ T cells. (C) Progression-free survival and (D) overall survival in Cat 1 (bold line), Cat 2 (thin dotted line), Cat 3 (thin line) and Cat 4 (bold dotted line) patients. PD-1, programmed cell death 1; CD, cluster of differentiation; Cat, category.
Table IV.
Change of category following hydrogen gas treatment.
Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis
Authors: Hideo Baba, Junji Akagi
View Affiliations
Published online on: November 1, 2018



